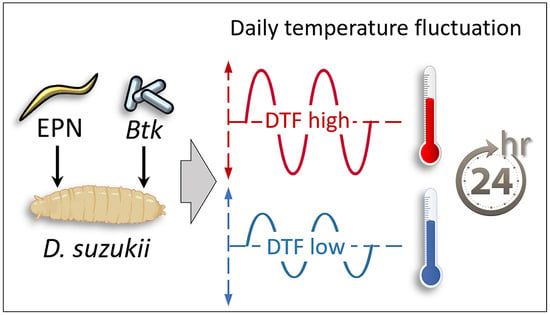
The Influence of Daily Temperature Fluctuation on the Efficacy of Bioinsecticides on Spotted Wing Drosophila Larvae
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Drosophila suzukii Stocks and Bioinsecticides
2.3. Biocontrol Assays
2.4. Data Analyses
3. Results
3.1. Effects of DTF on B. thuringiensis Administration
3.2. Effects of DTF on S. carpocapsae Administration
3.3. Effects of DTF on S. feltiae Administration
3.4. Effects of DTF on H. bacteriophora Administration
3.5. Comparison of the Maximum Efficacy of Bioinsecticides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sánchez-Lugo, A.; Berrisford, P.; Morice, C.; Argüez, A. Temperature [in State of the Climate in 2018]. Bull. Am. Meteorol. Soc. 2018, 99, S11–S12. [Google Scholar]
- IPCC. 2022. Available online: https://www.ipcc.ch/report/sixth-assessment-report-cycle/ (accessed on 15 July 2022).
- Somasekhar, N.; Prasad, J.S. Nematological considerations in addressing impact of climate change on agriculture. In Proceedings of the National Symposium on Innovations in Nematological Research, Tamil Nadu Agricultural University, Coimbatore, India, 23–25 February 2010. [Google Scholar]
- Kocmánková, E.; Trnka, M.; Juroch, J.; Dubrovský, M.; Semerádová, D.; Možný, M.; Žalud, Z.; Pokorný, R.; Lebeda, A. Impact of climate change on the occurrence and activity of harmful organisms. Plant Prot. Sci. 2010, 45, S48–S52. [Google Scholar] [CrossRef] [Green Version]
- Castex, V.; Beniston, M.; Calanca, P.; Fleury, D.; Moreau, J. Pest management under climate change: The importance of understanding tritrophic relations. Sci. Total Environ. 2018, 616–617, 397–407. [Google Scholar] [CrossRef]
- Deutsch, C.A.; Tewksbury, J.J.; Tigchelaar, M.; Battisti, D.S.; Merrill, S.C.; Huey, R.B.; Naylor, R.L. Increase in crop losses to insect pests in a warming climate. Science 2018, 361, 916–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojda, I. Temperature stress and insect immunity. J. Therm. Biol. 2017, 68, 96–103. [Google Scholar] [CrossRef]
- Ma, C.S.; Zhang, W.; Peng, Y.; Zhao, F.; Chang, X.Q.; Xing, K.; Zhu, L.; Ma, G.; Yang, H.P.; Rudolf, V.H.W. Climate warming promotes pesticide resistance through expanding overwintering range of a global pest. Nat. Commun. 2021, 12, e5351. [Google Scholar] [CrossRef] [PubMed]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić., D. The Impact of Climate Change on Agricultural Insect Pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Masood, N.; Akram, R.; Fatima, M.; Mubeen, M.; Hussain, S.; Shakeel, M.; Khan, N.; Adnan, M.; Wahid, A.; Noor Shah, A.; et al. Insect Pest Management Under Climate Change. In Building Climate Resilience in Agriculture; Jatoi, W.N., Mubeen, M., Ahmad, A., Cheema, M.A., Lin, Z., Hashmi, M.Z., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Outhwaite, C.L.; McCann, P.; Newbold, T. Agriculture and climate change are reshaping insect biodiversity worldwide. Nature 2022, 605, 97–102. [Google Scholar] [CrossRef]
- Chidawanyika, F.; Mudavanhu, P.; Nyamukondiwa, C. Biologically Based Methods for Pest Management in Agriculture under Changing Climates: Challenges and Future Directions. Insects 2012, 3, 1171–1189. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-B.; Yang, A.-P.; Zhang, G.-F.; Liu, W.-X.; Wan, F.-H. Effects of Simulated Heat Waves on Life History Traits of a Host Feeding Parasitoid. Insects 2019, 10, 419. [Google Scholar] [CrossRef]
- Bloomfield, J.P.; Williams, R.J.; Gooddy, D.C.; Cape, J.N.; Guha, P. Impacts of climate change on the fate and behaviour of pesticides in surface and groundwater. A UK perspective. Sci. Total Environ. 2006, 369, 163–177. [Google Scholar] [CrossRef]
- Nadal, M.; Marquès, M.; Mari, M.; Domingo, J.L. Climate change and environmental concentrations of POPs: A review. Environ. Res. 2015, 143, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, P.P.; Saha, S. Dynamics of pesticides under changing climatic scenario. Environ. Monit. Assess. 2020, 192, e814. [Google Scholar] [CrossRef] [PubMed]
- Delnat, V.; Verborgt, J.; Janssens, L.; Stoks, R. Daily temperature variation lowers the lethal and sublethal impact of a pesticide pulse due to a higher degradation rate. Chemosphere 2021, 263, e128114. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giles, K.L.; McCornack, B.P.; Royer, T.A.; Elliott, N.C. Incorporating biological control into IPM decision making. Curr. Opin. Insect Sci. 2017, 20, 84–89. [Google Scholar] [CrossRef]
- McCalla, K.A.; Keçeci, M.; Milosavljević, I.; Ratkowsky, D.A.; Hoddle, M.S. The Influence of Temperature Variation on Life History Parameters and Thermal Performance Curves of Tamarixia radiata (Hymenoptera: Eulophidae), a Parasitoid of the Asian Citrus Psyllid (Hemiptera: Liviidae). J. Econ. Entomol. 2019, 112, 1560–1574. [Google Scholar] [CrossRef] [PubMed]
- Milosavljević, I.; McCalla, K.A.; Ratkowsky, D.A.; Hoddle, M.S. Effects of Constant and Fluctuating Temperatures on Development Rates and Longevity of Diaphorencyrtus aligarhensis (Hymenoptera: Encyrtidae). J. Econ. Entomol. 2019, 112, 1062–1072. [Google Scholar] [CrossRef]
- Bahar, M.H.; Soroka, J.J.; Dosdall, L.M. Constant versus fluctuating temperatures in the interactions between Plutella xylostella (Lepidoptera: Plutellidae) and its larval parasitoid Diadegma insulare (Hymenoptera: Ichneumonidae). Environ. Entomol. 2021, 41, 1653–1661. [Google Scholar] [CrossRef]
- Colinet, H.; Sinclair, B.J.; Vernon, P.; Renault, D. Insects in fluctuating thermal environments. Annu. Rev. Entomol. 2015, 60, 123–140. [Google Scholar] [CrossRef]
- Rossi Stacconi, M.V.; Panel, A.; Baser, N.; Ioriatti, C.; Pantezzi, T.; Anfora, G. Comparative life history traits of indigenous Italian parasitoids of Drosophila suzukii and their effectiveness at different temperatures. Biol. Control. 2017, 112, 20–27. [Google Scholar] [CrossRef]
- Furlong, M.J.; Zalucki, M.P. Climate change and biological control: The consequences of increasing temperatures on host-parasitoid interactions. Curr. Opin. Insect Sci. 2017, 20, 39–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastore, M.; Quadroni, S.; Toscano, A.; Mottadelli, N.; Brivio, M.F. Susceptibility to entomopathogens and modulation of basal immunity in two insect models at different temperatures. J. Therm. Biol. 2019, 79, 15–23. [Google Scholar] [CrossRef]
- Cavigliasso, F.; Gatti, J.L.; Colinet, D.; Poirié, M. Impact of Temperature on the Immune Interaction between a Parasitoid Wasp and Drosophila Host Species. Insects 2021, 12, 647. [Google Scholar] [CrossRef]
- Vangansbeke, D.; Audenaert, J.; Nguyen, D.T.; Verhoeven, R.; Gobin, B.; Tirry, L.; De Clercq, P. Diurnal temperature variations affect development of an herbivorous arthropod pest and its predators. PLoS ONE 2015, 10, e0124898. [Google Scholar] [CrossRef] [Green Version]
- CABI. 2022. Available online: www.cabi.org/isc/datasheet/109283#tobiologyAndEcology (accessed on 15 July 2022).
- Cini, A.; Ioriatti, C.; Anfora, G. A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull. Insectol. 2012, 65, 149–160. [Google Scholar]
- Tochen, S.; Dalton, D.T.; Wiman, N.; Hamm, L.; Shearer, P.W.; Walton, V.M. Temperature related development and population parameters for Drosophila suzukii (Diptera: Drosophilidae) on cherry and blueberry. Environ. Entomol. 2014, 43, 501–510. [Google Scholar] [CrossRef] [Green Version]
- Ryan, G.D.; Emiljanowicz, L.; Wilkinson, F.; Kornya, M.; Newman, J.A. Thermal tolerances of the spotted-wing Drosophila Drosophila suzukii (Diptera: Drosophilidae). J. Econom. Entomol. 2016, 109, 746–752. [Google Scholar] [CrossRef] [Green Version]
- Zerulla, F.N.; Augel, C.; Zebitz, C.P.W. Oviposition activity of Drosophila suzukii as mediated by ambient and fruit temperature. PLoS ONE 2017, 11, e0187682. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Ramos, I.; Goméz-Casado, E.; Fernández, C.E.; González-Nunes, M. Reproductive potential and population increase of Drosophila suzukii at constant temperatures. Entomol. Gen. 2019, 39, 103–115. [Google Scholar] [CrossRef]
- Shawer, R. Chemical Control of Drosophila suzukii. In Drosophila suzukii Management; Garcia, F.R.M., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Introduction to Integrated Pest Management. Available online: http://www.epa.gov/ipm/introduction-integrated-pest-management (accessed on 20 August 2022).
- Integrated Pest Management (IPM). Available online: https://food.ec.europa.eu/plants/pesticides/sustainable-use-pesticides/integrated-pest-management-ipm_en (accessed on 19 August 2022).
- Guidelines on pesticide legislation. Available online: https://www.who.int/publications/i/item/9789241509671 (accessed on 20 August 2022).
- Garriga, A.; Mastore, M.; Morton, A.; Garcia-del-Pino, F.; Brivio, M.F. Immune response of Drosophila suzukii larvae to infection with the nematobacterial complex Steinernema carpocapsae-Xenorhabdus nematophila. Insects 2020, 11, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastore, M.; Caramella, S.; Quadroni, S.; Brivio, M.F. Drosophila suzukii Susceptibility to the Oral Administration of Bacillus thuringiensis, Xenorhabdus nematophila and Its Secondary Metabolites. Insects 2021, 12, 635. [Google Scholar] [CrossRef] [PubMed]
- Mastore, M.; Quadroni, S.; Brivio, M.F. Susceptibility of Drosophila suzukii larvae to the combined administration of the entomopathogens Bacillus thuringiensis and Steinernema carpocapsae. Sci. Rep. 2021, 11, e8149. [Google Scholar] [CrossRef] [PubMed]
- Mazzetto, F.; Marchetti, E.; Amiresmaeili, N.; Sacco, D.; Francati, S.; Jucker, C.; Dindo, M.L.; Lupi, D.; Tavella, L. Drosophila parasitoids in northern Italy and their potential to attack the exotic pest Drosophila suzukii. J. Pest Sci. 2016, 89, 837–850. [Google Scholar] [CrossRef]
- Panel, A.D.C.; Zeeman, L.; van der Sluis, B.J.; van Elk, P.; Pannebakker, B.A.; Wertheim, B.; Helsen, H.H.M. Overwintered Drosophila suzukii Are the Main Source for Infestations of the First Fruit Crops of the Season. Insects 2018, 9, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiman, N.G.; Walton, V.M.; Dalton, D.T.; Anfora, G.; Burrack, H.J.; Chiu, J.C.; Daane, K.M.; Grassi, A.; Miller, B.; Tochen, S.; et al. Integrating temperature-dependent life table data into a matrix projection model for Drosophila suzukii population estimation. PLoS ONE 2014, 9, e106909. [Google Scholar] [CrossRef]
- Verheyen, J.; Delnat, V.; Theys, C. Daily temperature fluctuations can magnify the toxicity of pesticides. Curr. Opin. Insect Sci. 2022, 51, e100919. [Google Scholar] [CrossRef]
- Cossentine, J.; Robertson, M.; Xu, D. Biological Activity of Bacillus thuringiensis in Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2019, 109, 1071–1078. [Google Scholar] [CrossRef]
- Warton, D.I.; Hui, F.K. The arcsine is asinine: The analysis of proportions in ecology. Ecology 2011, 92, 3–10. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Shearer, P.W.; West, J.D.; Walton, V.M.; Brown, P.H.; Svetec, N.; Chiu, J.C. Seasonal cues induce phenotypic plasticity of Drosophila suzukii to enhance winter survival. BMC Ecol. 2016, 16, e11. [Google Scholar] [CrossRef] [Green Version]
- Ørsted, M.; Lye, J.; Umina, P.A.; Maino, J.L. Global analysis of the seasonal abundance of the invasive pest Drosophila suzukii reveal temperature extremes determine population activity potential. Pest. Manag. Sci. 2021, 77, 4555–4563. [Google Scholar] [CrossRef] [PubMed]
- Eben, A.; Sporer, F.; Vogt, H.; Wetterauer, P.; Wink, M. Search for Alternative Control Strategies of Drosophila suzukii (Diptera: Drosophilidae): Laboratory Assays Using Volatile Natural Plant Compounds. Insects 2020, 11, 811. [Google Scholar] [CrossRef]
- Rossi Stacconi, M.V.; Amiresmaeili, N.; Biondi, A.; Carli, C.; Caruso, S.; Dindo, M.L.; Francati, S.; Gottardello, A.; Grassi, A.; Lupi, D.; et al. Host location and dispersal ability of the cosmopolitan parasitoid Trichopria drosophilae released to control the invasive spotted wing drosophila. Biol. Control. 2018, 117, 188–196. [Google Scholar] [CrossRef]
- Lee, J.C.; Wang, X.; Daane, K.M.; Hoelmer, K.A.; Isaacs, R.; Sial, A.A.; Walton, V.M. Biological Control of Spotted-Wing Drosophila (Diptera: Drosophilidae)—Current and Pending Tactics. J. Integr. Pest Manag. 2019, 10, e13. [Google Scholar] [CrossRef]
- Wraight, S.P.; Molloy, D.; Jamnback, H.; McCoy, P. Effects of temperature and instar on the efficacy of Bacillus thuringiensis var. israelensis and Bacillus sphaericus strain 1593 against Aedes stimulans larvae. J. Inv. Pathol. 1981, 38, 78–87. [Google Scholar] [CrossRef]
- Yilmaz, S.; Karabörklü, S.; Azizoğlu, U.; Ayvaz, A.; Akbulut, M.; Yildiz, M. Toxicity of native Bacillus thuringiensis isolates on the larval stages of pine processionary moth Thaumetopoea wilkinsoni at different temperatures. Turkish J. Agri. Forest. 2013, 37, e5. [Google Scholar] [CrossRef]
- Lysyk, T.J.; Selinger, L.B. Effects of temperature on mortality of larval stable fly (Diptera: Muscidae) caused by five isolates of Bacillus thuringiensis. J. Econ. Entomol. 2012, 105, 732–737. [Google Scholar] [CrossRef] [Green Version]
- Matter, M.M.; Gesraha, M.A. The role of temperature in enhancing the insecticidal activity of Bacillus thuringiensis against Spodoptera littoralis larvae. Arch. Phytopathol. Plant Protec. 2012, 45, 360–365. [Google Scholar] [CrossRef]
- Glazer, I. Survival and efficacy of Steinernema carpocapsae in an exposed environment. Biocontrol Sci. Technol. 1992, 2, 101–107. [Google Scholar] [CrossRef]
- Bobardt, S.D.; Dillman, A.R.; Nair, M.G. The Two Faces of Nematode Infection: Virulence and Immunomodulatory Molecules from Nematode Parasites of Mammals, Insects and Plants. Front. Microbiol. 2020, 11, e577846. [Google Scholar] [CrossRef] [PubMed]
- Levy, N.; Faigenboim, A.; Salame, L.; Molina, C.; Ehlers, R.U.; Glazer, I.; Ment, D. Characterization of the phenotypic and genotypic tolerance to abiotic stresses of natural populations of Heterorhabditis bacteriophora. Sci. Rep. 2020, 10, e10500. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, J.; Salame, L.; Nasser, A.; Glazer, I.; Ment, D. Survival and efficacy of entomopathogenic nematodes on exposed surfaces. Sci. Rep. 2022, 12, e4629. [Google Scholar] [CrossRef]
- Garriga, A.; Morton, A.; Garcia-del-Pino, F. Is Drosophila suzukii as susceptible to entomopathogenic nematodes as Drosophila melanogaster? J. Pest Sci. 2017, 91, 789–798. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.; Audsley, N. Further Screening of Entomopathogenic Fungi and Nematodes as Control Agents for Drosophila suzukii. Insects 2016, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Hübner, A.; Englert, C.; Herz, A. Effect of entomopathogenic nematodes on different developmental stages of Drosophila suzukii in and outside fruits. BioControl 2017, 62, 669–680. [Google Scholar] [CrossRef]
- Kim, D.; Alvarez, M.; Lechuga, L.M.; Louis, M. Species-specific modulation of food-search behavior by respiration and chemosensation in Drosophila larvae. Elife 2017, 6, e27057. [Google Scholar] [CrossRef]
- St Leger, R.J. Insects and their pathogens in a changing climate. J. Invertebr. Pathol. 2021, 184, e107644. [Google Scholar] [CrossRef]
- Řeřicha, M.; Dobeš, P.; Knapp, M. Changes in haemolymph parameters and insect ability to respond to immune challenge during overwintering. Ecol. Evol. 2021, 11, 4267–4275. [Google Scholar] [CrossRef]
- Rapporto Idro Meteo Clima Emilia-Romagna. Available online: https://www.arpae.it/it/notizie/pubblicato-il-rapporto-idrometeoclima-emilia-romagna-del-2021 (accessed on 20 January 2022).






| DF | χ2 | p | DF | χ2 | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| Btk | Logistic regression | 35 | 905 | <0.0001 | Sf | Logistic regression | 23 | 196 | <0.0001 |
| Temperature | 2 | 90 | <0.0001 | Temperature | 1 | 86 | <0.0001 | ||
| Concentration | 5 | 1268 | <0.0001 | Concentration | 5 | 191 | <0.0001 | ||
| Time | 1 | 232 | <0.0001 | Time | 1 | 274 | <0.0001 | ||
| Temp*Conc | 10 | 158 | <0.0001 | Temp*Conc | 5 | 104 | <0.0001 | ||
| Temp*Time | 2 | 110 | <0.0001 | Temp*Time | 1 | 161 | <0.0001 | ||
| Conc*Time | 5 | 103 | <0.0001 | Conc*Time | 5 | 31 | <0.0001 | ||
| Temp*Conc*Time | 10 | 74 | <0.0001 | Temp*Conc*Time | 5 | 41 | <0.0001 | ||
| Sc | Logistic regression | 35 | 793 | <0.0001 | Hb | Logistic regression | 23 | 88 | <0.0001 |
| Temperature | 2 | 40 | <0.0001 | Temperature | 1 | 113 | <0.0001 | ||
| Concentration | 5 | 1487 | <0.0001 | Concentration | 5 | 236 | <0.0001 | ||
| Time | 1 | 116 | <0.0001 | Time | 1 | 189 | <0.0001 | ||
| Temp*Conc | 10 | 701 | <0.0001 | Temp*Conc | 5 | 64 | <0.0001 | ||
| Temp*Time | 2 | 42 | <0.0001 | Temp*Time | 1 | 87 | <0.0001 | ||
| Conc*Time | 5 | 15 | 0.008 | Conc*Time | 5 | 78 | <0.0001 | ||
| Temp*Conc*Time | 10 | 112 | <0.0001 | Temp*Conc*Time | 5 | 56 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastore, M.; Quadroni, S.; Rezzonico, A.; Brivio, M.F. The Influence of Daily Temperature Fluctuation on the Efficacy of Bioinsecticides on Spotted Wing Drosophila Larvae. Insects 2023, 14, 43. https://doi.org/10.3390/insects14010043
Mastore M, Quadroni S, Rezzonico A, Brivio MF. The Influence of Daily Temperature Fluctuation on the Efficacy of Bioinsecticides on Spotted Wing Drosophila Larvae. Insects. 2023; 14(1):43. https://doi.org/10.3390/insects14010043
Chicago/Turabian StyleMastore, Maristella, Silvia Quadroni, Alberto Rezzonico, and Maurizio Francesco Brivio. 2023. "The Influence of Daily Temperature Fluctuation on the Efficacy of Bioinsecticides on Spotted Wing Drosophila Larvae" Insects 14, no. 1: 43. https://doi.org/10.3390/insects14010043
APA StyleMastore, M., Quadroni, S., Rezzonico, A., & Brivio, M. F. (2023). The Influence of Daily Temperature Fluctuation on the Efficacy of Bioinsecticides on Spotted Wing Drosophila Larvae. Insects, 14(1), 43. https://doi.org/10.3390/insects14010043