Bee Assemblage in the Southern Chihuahuan Desert: The Role of Season, Year, and Trap Color in Abundance
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
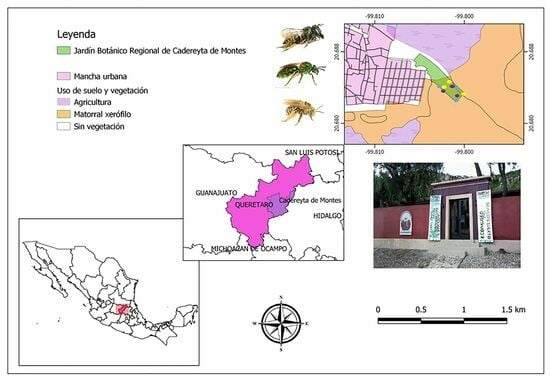
2.1. Study Area
2.2. Bee Capture
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Resasco, J.; Chacoff, N.P.; Vázquez, D.P. Plant–pollinator interactions between generalists persist over time and space. Ecology 2021, 102, e03359. [Google Scholar] [CrossRef]
- Hadrava, J.; Talašová, A.; Straka, J.; Benda, D.; Kazda, J.; Klečka, J. A comparison of wild bee communities in sown flower strips and semi-natural habitats: A pollination network approach. Insect Conserv. Divers. 2022, 15, 312–324. [Google Scholar] [CrossRef]
- Sagot, P.; Borrell, E.V.; Mérida-Rivas, J.A. Abejas y agricultura: Cuando la diversidad es necesidad. Ecofronteras 2021, 25, 10–13. [Google Scholar]
- Orr, M.C.; Hughes, A.C.; Chesters, D.; Pickering, J.; Zhu, C.; Ascher, J.S. Global Patterns and Drivers of Bee Distribution. Curr. Biol. 2021, 31, 451–458. [Google Scholar] [CrossRef]
- Danforth, B.N.; Minckley, R.L.; Neff, J.L.; Fawcett, F. The Solitary Bees: Biology, Evolution, Conservation; Princeton University Press: Princeton, NJ, USA, 2019. [Google Scholar]
- Potts, S.; Imperatriz-Fonseca, V.; Ngo, H.; Biesmeijer, J.; Breeze, T.; Dicks, L.; Garibaldi, L.; Hill, R.; Settele, J.; Vanbergen, A.; et al. Summary for Policymakers of the Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on Pollinators, Pollination and Food Production; Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2016; p. 36. [Google Scholar]
- FAO. Blending Tradition and Science to Protect Pollinators. Available online: http://www.fao.org/in-action/blending-tradition-and-science-toprotect-pollinators/es/ (accessed on 18 February 2017).
- Wagner, D.L.; Grames, E.M.; Forister, M.L.; Berenbaum, M.R.; Stopak, D. Insect decline in the Anthropocene: Death by a thousand cuts. Proc. Natl. Acad. Sci. USA 2021, 118, e2023989118. [Google Scholar] [CrossRef]
- IUCN. The ICN Red List of Threatened Species. Version 2022-1. Available online: https://www.iucnredlist.org. (accessed on 29 September 2022).
- Hristov, P.; Neov, B.; Shumkova, R.; Palova, N. Significance of Apoidea as main pollinators. ecological and economic impact and implications for human nutrition. Diversity 2020, 12, 280. [Google Scholar] [CrossRef]
- Vasiliev, D.; Greenwood, S. Pollinator biodiversity and crop pollination in temperate ecosystems, implications for national pollinator conservation strategies: Mini review. Sci. Total Environ. 2020, 744, 140880. [Google Scholar] [CrossRef]
- Patel, S. Reviewing the prospects of Opuntia pears as low cost functional foods. Rev. Environ. Sci. 2013, 12, 223–234. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Winfree, R.; Bartomeus, I.; Cariveau, D.P. Native pollinators in anthropogenic habitats. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 1–22. [Google Scholar] [CrossRef]
- Lasway, J.V.; Steffan-Dewenter, I.; Njovu, H.K.; Kinabo, N.R.; Eardley, C.; Pauly, A.; Peters, M.K. Positive effects of low grazing intensity on East African bee assemblages mediated by increases in floral resources. Biol. Conserv. 2022, 267, 109–490. [Google Scholar] [CrossRef]
- Goulson, D.; Lye, G.C.; Darvill, B. Decline and conservation of bumble bees. Annu. Rev. Entomol. 2008, 53, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Cane, J.H.; Minckley, R.L.; Kervin, L.J.; Roulston, T.A.H.; Williams, N.M. Complex responses within a desert bee guild (Hymenoptera: Apiformes) to urban habitat fragmentation. Ecol. Appl. 2006, 16, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Medina-Flores, C.A.; Guzmán-Novoa, E.; Hamiduzzaman, M.M.; Aréchiga-Flores, C.F.; López-Carlos, M.A. Africanized honey bees (Apis mellifera) have low infestation levels of the mite Varroa destructor in different ecological regions in Mexico. Genet. Mol. Res. 2014, 13, 7282–7293. [Google Scholar] [CrossRef] [PubMed]
- Valido, A.; Rodríguez-Rodríguez, M.C.; Jordano, P. Interacciones entre plantas y polinizadores en el Parque Nacional del Teide: Consecuencias ecológicas de la introducción masiva de la abeja doméstica, Apis mellifera (Apidae). In Proyectos de Investigacion en Parques Nacionales; Sanz, L.R., Nistal, B.A., Eds.; Organismo Autónomo Parques Nacionales: Madrid, Spain, 2011; pp. 205–232. [Google Scholar]
- Martin-Culma, N.Y.; Arenas-Suárez, N.E.A.-S.E. Daño colateral en abejas por la exposición a pesticidas de uso agrícola. Entramado 2018, 14, 232–240. [Google Scholar] [CrossRef]
- Drossart, M.; Gérard, M. Beyond the decline of wild bees: Optimizing conservation measures and bringing together the actors. Insects 2020, 11, 649. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecological Methodology; Harper & Row: New York, NY, USA, 1989; p. 739. [Google Scholar]
- Caswell, H. Matrix Population Models: Construction, Analysis, and Interpretation, 2nd ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2001. [Google Scholar]
- Gotelli, N.J.; Colwell, R.K. Estimating Species Richness. In Biological Diversity: Frontiers in Measurement and Assessment; Oxford University Press: Oxford, UK, 2011; pp. 39–54. [Google Scholar]
- White, E.R. Minimum Time Required to Detect Population Trends: The Need for Long-Term Monitoring Programs. BioScience 2019, 69, 40–46. [Google Scholar] [CrossRef]
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen; Universidad Nacional Autónoma de México (UNAM): Mexico City, Mexico, 2004; p. 90. [Google Scholar]
- INEGI. Cuaderno Estadístico Municipal Cadereyta de Montes; Querétaro de Arteaga: Aguascalientes, Mexico, 2000. [Google Scholar]
- de la Cruz, Y.H.U.; Aguilar, B.M.; Martínez, E.S.; Carrillo-Ángeles, I.; Martínez, M.M.H.; Vázquez, H.G.A. Estrategias de Conservación in situ para Rehabilitar los Paisajes del Semidesierto Queretano-Hidalguense; Boletín Amaranto, Asociación Mexicana de Jardines Botánicos: Querétaro, Mexico, 2018; p. 33. [Google Scholar]
- Hailen, U.d.l.C.; Maruri, A.B.; Carrillo, A.I.G.; Sánchez, E. Estrategias para la restauración con un enfoque agroforestal de áreas degradadas circunscritas por zona súrbanas en la región semiárida de Querétaro. Nthe 2004, 8, 3–9. [Google Scholar]
- CEA-CONCYTEQ, W.E. Forecast for Cadereyta, Queretaro. Available online: https://www.wunderground.com/dashboard/pws/IQUERETA15 (accessed on 25 November 2020).
- Ramírez-Freire, L.; Alanís Flores, G.; Ayala Barajas, R.; Velazco Macías, C.; Favela Lara, S. El uso de platos trampa y red entomológica en la captura de abejas nativas en el estado de Nuevo León, México. Acta Zool. Mex. 2014, 30, 508–538. [Google Scholar] [CrossRef]
- Fortel, L.; Henry, M.; Guilbaud, L.; Guirao, A.L.; Kuhlmann, M.; Mouret, H.; Rollin, O.; Vaissière, B.E. Decreasing Abundance, Increasing Diversity and Changing Structure of the Wild Bee Community (Hymenoptera: Anthophila) along an Urbanization Gradient. PLoS ONE 2014, 9, e104679. [Google Scholar] [CrossRef]
- Schiestl, F.P.; Johnson, S.D. Pollinator-mediated evolution of floral signals. Trends Ecol. Evol. 2003, 28, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Vrdoljak, S.M.; Samways, M.J. Optimising coloured pan traps to survey flower visiting insects. J. Insect Conserv. 2012, 16, 345–354. [Google Scholar] [CrossRef]
- Shapiro, L.H.; Tepedino, V.J.; Minckley, R.L. Bowling for bees: Optimal sample number for “bee bowl” sampling transects. J. Insect Conserv. 2014, 18, 1105–1113. [Google Scholar] [CrossRef]
- Sibly, R.M.; Hone, J. Population growth rate and its determinants: An overview. Philos. Trans. R. Soc. B 2002, 357, 1153–1170. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Prentis Hall: Englewood Cliffs, NJ, USA, 1999. [Google Scholar]
- Crawley, M. The R Book, 2nd ed.; John Wiley and Sons Ltd.: Sussex, UK, 2013. [Google Scholar]
- Ortiz Sánchez, F.J.; Aguirre Segura, A. Estructura y dinámica estacional de una comunidad de Apoidea (Hymenoptera) en Almería. EOS Rev. Esp. Entomol. 1991, 67, 3–22. [Google Scholar]
- Roubik, D.W. Ecological impact on native bees by the invasive Africanized honey bee. Acta Biol. Colomb. 2009, 14, 115–124. [Google Scholar]
- Granados-Sánchez, D.; Sánchez-González, A.; Granados Victorino, R.L.; Borja de la Rosa, A. Ecología de la vegetación del Desierto Chihuahuense. RCHSCFA 2011, 17, 111–130. [Google Scholar] [CrossRef]
- Minckley, R.L.; Cane, J.H.; Kervin, L.; Roulston, T. Spatial predictability and resource specialization of bees (Hymenoptera: Apoidea) at a superabundant, widespread resource. Biol. J. Linn. Soc. 1999, 67, 119–147. [Google Scholar] [CrossRef]
- Packer, L.; Jessome, V.; Lockerbie, C.; Sampson, B. The phenology and social biology of four sweat bees in a marginal environment: Cape Breton Island. Can. J. Zool. 1989, 67, 2871–2877. [Google Scholar] [CrossRef]
- Neff, J.L.; Simpson, B.B. Partial bivoltinism in a ground-nesting bee: The biology of Diadasia rinconis in Texas (Hymenoptera, Anthophoridae). J. Kans. Entomol. Soc. 1992, 65, 377–392. [Google Scholar]
- Portman, Z.M.; Cariveau, D.P. The State of Bee Monitoring in the United States: A Call to Refocus Away From Bowl Traps and Towards More Effective Methods. Ann. Entomol. Soc. Am. 2020, 113, 337–342. [Google Scholar] [CrossRef]
- Riggs, E.L.; Baranski, C.; Schaetz, O.M.; Garrison, G.; Collazo, J.A.; Youngsteadt, E. Estimating bee abundance: Can mark-recapture methods validate common sampling protocols? Apidologie 2022, 53, 10. [Google Scholar] [CrossRef]
- Boyer, K.J.; Fragoso, F.P.; Dieterich Mabin, M.E.; Brunet, J. Netting and pan traps fail to identify the pollinator guild of an agricultural crop. Sci. Rep. 2020, 10, 13819. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, I. Population dynamics of the solitary digger bee Andrena vaga Panzer (Hymenoptera, Andrenidae) studied using mark-recapture and nest counts. Popul. Ecol. 2003, 45, 197–204. [Google Scholar] [CrossRef]
- Steffan-Dewenter, I.; Schiele, S. Nest-site fidelity, body weight and population size of the red mason bee, Osmia rufa (Hymenoptera: Megachilidae), evaluated by mark-recapture experiments. Entomol. Gen. 2004, 27, 123–131. [Google Scholar] [CrossRef]
- Michener, C.D. The Bees of the World, 2nd ed.; The Johns Hopkins University Press: Baltimore, MD, USA, 2007. [Google Scholar]
- Cano-Salgado, A.; Zavala-Hurtado, J.A.; Orozco-Segovia, A.; Valverde-Valdés, M.T.; Pérez-Rodríguez, P. Composición y abundancia del banco de semillas en una región semiárida del trópico mexicano: Patrones de variación espacial y temporal. Rev. Mex. Biodivers. 2012, 83, 437–446. [Google Scholar] [CrossRef]
- Carevic, F.S. Hacia una integración de los rasgos ecofisiológicos de las plantas para la conservación de especies en peligro en ecosistemas sometidos a estrés hídrico. Idesia (Arica) 2016, 34, 33–38. [Google Scholar] [CrossRef]
- Pickett, S.T.A.; Ostfeld, R.S.; Shachak, M.; Likens, G.E. The Ecological Basis of Conservation. Heterogeneity, Ecosystems and Biodiversity; Springer: New York, NY, USA, 1997. [Google Scholar]


| Years | Temperature (°C) | Precipitation (mm) | Wind Speed (km/h) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average | sd | R | p-Value | Average | sd | R | p-Value | Average | sd | R | p-Value | |
| 2015 | 16.1 | 2.17 | 0.65 | 0.02 | 24.3 | 30.16 | 0.42 | 0.17 | 16.9 | 3.44 | −0.15 | 0.63 |
| 2016 | 16.4 | 2.92 | 0.82 | 0.001 | 33.3 | 34.31 | 0.19 | 0.54 | 16.7 | 1.86 | 0.58 | 0.05 |
| 2018 | 16.8 | 2.84 | 0.57 | 0.05 | 17.9 | 34.9 | 0.42 | 0.18 | 16.9 | 2.31 | 0.3 | 0.34 |
| 2019 | 17.8 | 2.64 | 0.83 | 0.001 | 17.1 | 22.14 | 0.16 | 0.62 | 16.1 | 1.8 | 0.55 | 0.07 |
| Family | Genus and Species | N15 | 15 | σ15 | D15 | N16 | 16 | σ16 | D16 | N18 | 18 | σ18 | D18 | N19 | 19 | σ19 | D2019 | Ntotal | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Andrenidae | Macrotera sinaloana (Timberlake, 1958) | 89 | 7.42 | 4.08 | 12.71 | 71 | 5.92 | 3.78 | 10.14 | 88 | 7.33 | 4.98 | 12.57 | 99 | 8.25 | 3.79 | 14.14 | 347 | 12.39 |
| Apidae | Apis mellifera (Linnaeus, 1758) | 161 | 13.42 | 5.38 | 23.00 | 104 | 8.67 | 3.60 | 14.86 | 63 | 5.25 | 4.54 | 9.00 | 91 | 7.58 | 3.85 | 13.00 | 419 | 14.96 |
| Bombus pensylvanicus (De Geer, 1773) | 12 | 1.00 | 2.37 | 1.71 | 9 | 0.75 | 0.97 | 1.29 | 0 | 0.00 | 0.00 | 0.00 | 0 | 0.00 | 0.00 | 0.00 | 21 | 0.75 | |
| Diadasia sp. | 50 | 4.17 | 2.98 | 7.14 | 42 | 3.50 | 3.92 | 6.00 | 42 | 3.50 | 2.71 | 6.00 | 51 | 4.25 | 3.33 | 7.29 | 185 | 6.61 | |
| Melissodes sp. | 0 | 0.00 | 0.00 | 0.00 | 20 | 1.67 | 1.92 | 2.86 | 15 | 1.25 | 1.96 | 2.14 | 28 | 2.33 | 2.50 | 4.00 | 63 | 2.25 | |
| Halictidae | Augochlorella sp. | 67 | 5.58 | 3.37 | 9.57 | 54 | 4.50 | 3.18 | 7.71 | 51 | 4.25 | 3.36 | 7.29 | 80 | 6.67 | 3.55 | 11.43 | 252 | 9.00 |
| Lasioglossum (Dialictus spp.) complex | 77 | 6.42 | 3.63 | 11.00 | 91 | 7.58 | 3.32 | 13.00 | 92 | 7.67 | 4.96 | 13.14 | 110 | 9.17 | 4.39 | 15.71 | 370 | 13.21 | |
| Megachilidae | Lithurgus littoralis (Cockerell, 1917) | 0 | 0.00 | 0.00 | 0.00 | 44 | 3.67 | 4.31 | 6.29 | 42 | 3.50 | 4.15 | 6.00 | 49 | 4.08 | 4.06 | 7.00 | 135 | 4.82 |
| Megachile sp. | 26 | 2.17 | 2.82 | 3.56 | 23 | 1.92 | 2.39 | 3.29 | 15 | 1.25 | 1.42 | 2.14 | 24 | 2.00 | 1.91 | 3.43 | 88 | 3.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munguia-Soto, E.O.; Golubov, J.; Mandujano, M.C. Bee Assemblage in the Southern Chihuahuan Desert: The Role of Season, Year, and Trap Color in Abundance. Insects 2023, 14, 875. https://doi.org/10.3390/insects14110875
Munguia-Soto EO, Golubov J, Mandujano MC. Bee Assemblage in the Southern Chihuahuan Desert: The Role of Season, Year, and Trap Color in Abundance. Insects. 2023; 14(11):875. https://doi.org/10.3390/insects14110875
Chicago/Turabian StyleMunguia-Soto, Esteban O., Jordan Golubov, and María C. Mandujano. 2023. "Bee Assemblage in the Southern Chihuahuan Desert: The Role of Season, Year, and Trap Color in Abundance" Insects 14, no. 11: 875. https://doi.org/10.3390/insects14110875
APA StyleMunguia-Soto, E. O., Golubov, J., & Mandujano, M. C. (2023). Bee Assemblage in the Southern Chihuahuan Desert: The Role of Season, Year, and Trap Color in Abundance. Insects, 14(11), 875. https://doi.org/10.3390/insects14110875