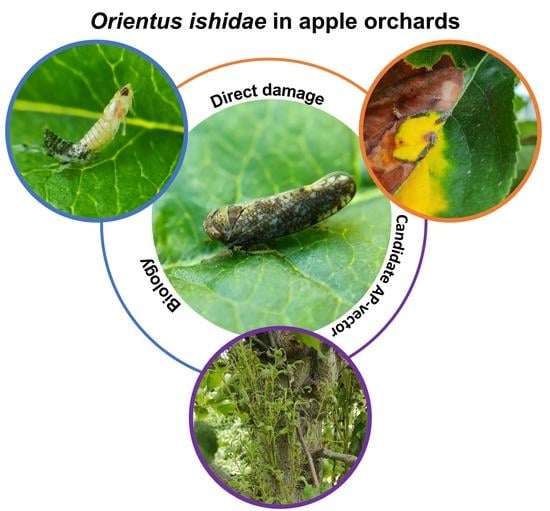
Orientus ishidae (Hemiptera: Cicadellidae): Biology, Direct Damage and Preliminary Studies on Apple Proliferation Infection in Apple Orchard
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Apple Orchards Description
2.2. Oviposition Site and Hatching Dynamics
2.3. Field Phenology and Flight Activities
2.4. Damage Characterisation and Quantification
2.5. AP Phytoplasma Infection
2.6. Data Analysis
3. Results
3.1. Oviposition Site and Hatching Dynamics
3.2. Field Phenology
3.3. Flight Activities
3.4. Damage Characterisation
3.5. AP Infectivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanders, J.G.; DeLong, D.M. Eight New “Jassids” from the Eastern United States. Ann. Entomol. Soc. Am. 1919, 12, 231–237. [Google Scholar] [CrossRef]
- Felt, E.P.; Bromley, S.W. New and Unusual Shade Tree Pests. J. Econ. Entomol. 1941, 34, 383–386. [Google Scholar] [CrossRef]
- Guglielmino, A. Observations on the genus Orientus (Rhynchota Cicadomorpha Cicadellidae) and description of a new species: O. amurensis n. sp. from Russia (Amur Region and Maritime Territory) and China (Liaoning Province). Marburger. Entomologische. Publikationen. 2005, 3, 99–110. [Google Scholar]
- Blaser, S.C. Invasion Genetics and Development of Rapid Diagnostics of Insect Pests on Traded Plants. Ph.D. Thesis, University of Basel, Basel, Switzerland, 2019. [Google Scholar]
- Johnson, M.; Freytag, P. Leafhoppers (Homoptera: Cicadellidae) on pin oak in Kentucky. J. Kans. Entomol. Soc. 2001, 74, 83–91. [Google Scholar]
- Nickel, H. First addendum to the Leafhoppers and Planthoppers of Germany (Hemiptera: Auchenorrhyncha). Cicadina 2010, 11, 107–122. [Google Scholar] [CrossRef]
- Koczor, S.; Bagarus, A.K.; Karap, A.K.; Varga, Á.; Orosz, A. A Rapidly spreading potential pest, Orientus ishidae identified in Hungary. Bull. Insectology 2013, 66, 221–224. [Google Scholar]
- Mazzoni, V. Contribution to the knowledge of the Auchenorrhyncha (Hemiptera Fulgoromorpha and Cicadomorpha) of Tuscany (Italy). Redia 2005, 88, 85–102. [Google Scholar] [CrossRef]
- Lessio, F.; Picciau, L.; Gonella, E.; Mandrioli, M.; Tota, F.; Alma, A. The Mosaic Leafhopper Orientus ishidae: Host Plants, Spatial Distribution, Infectivity, and Transmission of 16SrV Phytoplasmas to Vines. Bull. Insectology 2016, 69, 277–289. [Google Scholar]
- Oppedisano, T. New Insights into the Biology and Ecology of the Insect Vectors of Apple Proliferation for the Development of Sustainable Control Strategies. Ph.D. Thesis, University of Molise, Campobasso, Italy, 2017. [Google Scholar]
- Garman, P.; Townsend, J.F. Control of Apple Insects: Bulletin 552; The Connecticut Agricultural: New Haven, CT, USA, 1952; pp. 44–45. [Google Scholar]
- Lešnik, M.; Seljak, G.; Vajs, S. Populacijska dinamika škržatka Orientus ishidae Matsumura v nasadih jablan v letih 2015 in 2016. In Proceedings of the 13th Slovenian Conference on Plant Protection with International Participation, Rimske Toplice, Slovenia, 7–8 March 2017; pp. 39–46. [Google Scholar]
- Gaffuri, F.; Sacchi, S.; Cavagna, B. First Detection of the Mosaic Leafhopper, Orientus ishidae, in Northern Italian Vineyards Infected by the Flavescence Dorée Phytoplasma. New Dis. Rep. 2011, 24, 22. [Google Scholar] [CrossRef] [Green Version]
- Mehle, N.; Seljak, G.; Rupar, M.; Ravnikar, M.; Dermastia, M. The First Detection of a Phytoplasma from the 16SrV (Elm Yellows) Group in the Mosaic Leafhopper Orientus ishidae. New Dis. Rep. 2010, 22, 11. [Google Scholar] [CrossRef] [Green Version]
- Trivellone, V.; Knop, E.; Turrini, T.; Andrey, A.; Humbert, J.-Y.; Kunz, G. New and Remarkable Leafhoppers and Planthoppers (Hemiptera: Auchenorrhyncha) from Switzerland. Mitt. Schweiz. Entomol. Ges. 2015, 88, 273–284. [Google Scholar] [CrossRef]
- Valley, K.R.; Wheeler, A.G. Leafhoppers (Hemiptera: Cicadellidae) associated with ornamental honey locust: Seasonal history, habits, and descriptions of eggs and fifth instars. Ann. Entomol. Soc. Am. 1985, 78, 709–716. [Google Scholar] [CrossRef]
- Desqué, D.; Salar, P.; Danet, J.L.; Lusseau, T.; Garcion, C.; Moreau, E.; Dubus, C.; Dureuil, J.; Delbac, L.; Binet, D.; et al. Impact of Orientus ishidae on “Flavescence Dorée” emergence in the vineyards of riparian ecosystems. Phytopathog. Mollicutes 2019, 9, 69–70. [Google Scholar] [CrossRef]
- Lessio, F.; Bocca, F.; Alma, A. Development, spatial distribution, and presence on grapevine of nymphs of Orientus ishidae (Hemiptera: Cicadellidae), a new vector of flavescence dorée phytoplasmas. J. Econ. Entomol. 2019, 112, 2558–2564. [Google Scholar] [CrossRef]
- Parise, G. Notes on the biology of Orientus ishidae (Matsumura, 1902) in Piedmont (Italy) (Hemiptera: Cicadellidae: Deltocephalinae). Cicadina 2017, 17, 19–28. [Google Scholar] [CrossRef]
- Casati, P.; Quaglino, F.; Tedeschi, R.; Spiga, F.M.; Alma, A.; Spadone, P.; Bianco, P.A. Identification and molecular characterization of ‘Candidatus Phytoplasma mali’ Isolates in North-Western Italy. J. Phytopathol. 2010, 158, 81–87. [Google Scholar] [CrossRef]
- Rosenberger, D.A. Leafhopper vectors of the peach X-disease pathogen and its seasonal transmission from chokecherry. Phytopathology 1978, 68, 782. [Google Scholar] [CrossRef]
- Davis, R.E.; Zhao, Y.; Dally, E.L.; Lee, I.M.; Jomantiene, R.; Douglas, S.M. “Candidatus Phytoplasma pruni”, A novel taxon associated with x-disease of stone fruits, Prunus spp.: Multilocus characterization based on 16S RRNA, SecY, and ribosomal protein genes. Int. J. Syst. Evol. Microbiol. 2013, 63, 766–776. [Google Scholar] [CrossRef]
- Baldessari, M.; Dalmaso, G.; Mazzoni, V.; Mori, N. Infestazioni su melo in Trentino di Orientus ishidae. Inf. Agrar. 2020, 30, 60–61. [Google Scholar]
- “Candidatus Phytoplasma mali” (PHYPMA)[Overview]|EPPO Global Database. Available online: https://gd.eppo.int/taxon/PHYPMA (accessed on 30 November 2022).
- Janik, K.; Barthel, D.; Oppedisano, T.; Anfora, G. Apple Proliferation: A Joint Review; Fondazione Edmund Mach and Laimburg Research Centre: San Michele all’Adige, Italy, 2020; ISBN 9788878430549. [Google Scholar]
- Frisinghelli, C.; Delaiti, L.; Grando, M.S.; Forti, D.; Vindimian, M.E. Cacopsylla Costalis (Flor 1861), as a Vector of Apple Proliferation in Trentino. J. Phytopathol. 2000, 148, 425–431. [Google Scholar] [CrossRef]
- Tedeschi, R.; Alma, A. Transmission of apple proliferation phytoplasma by Cacopsylla melanoneura (Homoptera: Psyllidae). J. Econ. Entomol. 2004, 97, 8–13. [Google Scholar] [CrossRef]
- Tedeschi, R.; Alma, A. Fieberiella florii (Homoptera: Auchenorrhyncha) as a Vector of “Candidatus Phytoplasma mali”. Plant Dis. 2006, 90, 284–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavan, F.; Cargnus, E.; Tacoli, F.; Zandigiacomo, P. Standardization and criticism of sampling procedures using sticky card traps: Monitoring sap-sucking insect pests and Anagrus atomus inhabiting European vineyards. Bull. Insectology 2021, 74, 291–306. [Google Scholar]
- Smart, C.D.; Schneider, B.; Blomquist, Ç.L.; Guerra, L.J.; Harrison, N.A.; Ahrens, U.; Lorenz, E.; Seemüller, K.H.; Kirkpatrick, B.C. Phytoplasma-Specific PCR Primers Based on Sequences of the 16S-23S RRNA Spacer Region. Appl. Environ. Microbiol. 1996, 62, 2988–2993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria|CiNii Research. Available online: https://cir.nii.ac.jp/crid/1574231874043578752 (accessed on 30 November 2022).
- Cribari-Neto, F.; Zeileis, A. Beta Regression in R. J. Stat. Softw. 2010, 34, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.; Weisberg, S. An R companion to Aapplied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2018; ISBN 978-1544336473. [Google Scholar]
- Baldessari, M.; (Fondazione Edmund Mach, San Michele all’Adige, Italy). Personal Communication, 2022.
- Elbert, A.; Haas, M.; Springer, B.; Thielert, W.; Nauen, R. Applied Aspects of Neonicotinoid Uses in Crop Protection. Pest Manag. Sci. 2008, 64, 1099–1105. [Google Scholar] [CrossRef]
- Chuche, J.; Thiéry, D. Cold Winter Temperatures Condition the Egg-Hatching Dynamics of a Grape Disease Vector. Naturwissenschaften 2009, 96, 827–834. [Google Scholar] [CrossRef] [Green Version]
- Chuche, J.; Thiéry, D. Biology and Ecology of the Flavescence Dorée Vector Scaphoideus titanus: A Review. Agron. Sustain. Dev. 2014, 34, 381–403. [Google Scholar] [CrossRef] [Green Version]
- Falzoi, S.; Lessio, F.; Spanna, F.; Alma, A. Influence of temperature on the embryonic and post-embryonic development of Scaphoideus titanus (Hemiptera: Cicadellidae), vector of grapevine Flavescence Dorée. Int. J. Pest Manag. 2014, 60, 246–257. [Google Scholar] [CrossRef]
- Mazzoni, V.; Cosci, F.; Lucchi, A.; Santini, L. Occurrence of leafhoppers (Auchenorrhyncha, Cicadellidae) in three vineyards of the Pisa district. In Proceedings of the IOBC/WPRS Bulletin, Ponte de Lima, Portugal, 3–7 March 2001; Volume 24, pp. 267–268. [Google Scholar]
- Lessio, F.; Tedeschi, R.; Pajoro, M.; Alma, A. Seasonal progression of sex ratio and phytoplasma infection in Scaphoideus titanus Ball (Hemiptera: Cicadellidae). Bull. Entomol. Res. 2009, 99, 377–383. [Google Scholar] [CrossRef] [Green Version]
- López Díez, J.J. Reproductive Strategy of the Leafhopper Orientus ishidae Matsumura (Hemiptera: Cicadellidae). Master’s Thesis, University of Ljubljana, Ljubljana, Slovenia, 2019. [Google Scholar]
- Hunt, R.E.; Nault, L.R. Roles of interplant movement, acoustic communication, and phototaxis in mate-location behavior of the leafhopper Graminella Nigrifrons. Behav. Ecol. Sociobiol. 1991, 28, 315–320. [Google Scholar] [CrossRef]
- Mazzoni, V.; Eriksson, A.; Anfora, G.; Lucchi, A.; Virant-Doberlet, M. Active Space and the Role of Amplitude in Plant-Borne Vibrational Communication. In Studying Vibrational Communication. Animal Ssignals and Communication; Cocroft, R., Gogala, M., Hill, P., Wessel, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 3, ISBN 978-3-662-43607-3. [Google Scholar]
- Miles, P.W. The Saliva of Hemiptera, In Advances in Insect Physiology; Treherne, J.E., Berridge, M.J., Wigglesworth, V.B., Eds.; Academic Press: London, UK, 1972; Volume 9, pp. 183–255. ISBN 9780120242092. [Google Scholar]
- Backus, E.A. Sensory systems and behaviours which mediate hemipteran plant-feeding: A taxonomic overview. J. Insect Physiol. 1988, 34, 151–165. [Google Scholar] [CrossRef]
- Backus, E.A.; Serrano, M.S.; Ranger, C.M. Mechanisms of hopperburn: An overview of insect taxonomy, behavior, and physiology. Annu. Rev. Entomol. 2005, 50, 125–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backus, E.A.; Habibi, J.; Yan, F.; Ellersieck, M. Stylet penetration by adult Homalodisca coagulata on grape: Electrical penetration graph waveform characterization, tissue correlation, and possible implications for transmission of Xylella fastidiosa. Ann. Entomol. Soc. Am. 2005, 98, 787–813. [Google Scholar] [CrossRef]
- Chuche, J.; Sauvion, N.; Thiéry, D. Mixed xylem and phloem sap ingestion in sheath-feeders as normal dietary behavior: Evidence from the leafhopper Scaphoideus titanus. J. Insect Physiol. 2017, 102, 62–72. [Google Scholar] [CrossRef]
- Cornara, D.; Garzo, E.; Morente, M.; Moreno, A.; Alba-Tercedor, J.; Fereres, A. EPG combined with micro-CT and video recording reveals new insights on the feeding behavior of Philaenus Spumarius. PLoS ONE 2018, 13, e0199154. [Google Scholar] [CrossRef]
- Jarausch, B.; Weintraub, P.G. Spread of phytoplasma diseases by insect vectors: An introduction. In Proceedings of the New Perspectives in Phytoplasma Disease Management, Barcelona, Spain, 22 March 2013; pp. 17–20. [Google Scholar]
- Jin, S.; Chen, Z.M.; Backus, E.A.; Sun, X.L.; Xiao, B. Characterization of EPG waveforms for the tea green leafhopper, Empoasca Vitis Göthe (Hemiptera: Cicadellidae), on tea plants and their correlation with stylet activities. J. Insect Physiol. 2012, 58, 1235–1244. [Google Scholar] [CrossRef]
- Miles, P.W. Aphid Saliva. Biol. Rev. 1999, 74, 41–85. [Google Scholar] [CrossRef]
- Nicholson, S.J.; Hartson, S.D.; Puterka, G.J. Proteomic analysis of secreted saliva from russian wheat aphid (Diuraphis noxia Kurd.) biotypes that differ in virulence to wheat. J. Proteomics 2012, 75, 2252–2268. [Google Scholar] [CrossRef]
- Sharma, A.; Khan, A.N.; Subrahmanyam, S.; Raman, A.; Taylor, G.S.; Fletcher, M.J. Salivary proteins of plant-feeding hemipteroids–implication in phytophagy. Bull. Entomol Res. 2014, 104, 117–136. [Google Scholar] [CrossRef]
- Van Bel, A.J.; Will, T. Functional evaluation of proteins in watery and gel saliva of aphids. Front. Plant. Sci. 2016, 7, 1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubia-Sanchez, E.; Suzuki, Y.; Arimura, K.; Miyamoto, K.; Matsumura, M.; Watanabe, T. Comparing Nilaparvata Lugens (Stal) and Sogatella Furcifera (Horvath) (Homoptera: Delphacidae) feeding effects on rice plant growth processes at the vegetative stage. Crop Prot. 2003, 22, 967–974. [Google Scholar] [CrossRef]
- Ab-Ghaffar, M.B.; Pritchard, J.; Ford-Lloyd, B. Brown planthopper (N. lugens Stal) feeding behaviour on rice germplasm as an indicator of resistance. PLoS ONE 2011, 6, e22137. [Google Scholar] [CrossRef]
- Cabauatan, P.Q.; Cabunagan, R.C.; Choi, I.R. Rice viruses transmitted by the brown planthopper Nilaparvata lugens Stål. In Planthoppers: New Threats to the Sustainability of Intensive Rice Production Systems in Asia; Heong, K.L., Hardy, B., Eds.; International Rice Research Istitute: Los Baños, Philippines, 2009; pp. 357–368. [Google Scholar]
- Lei, W.; Li, P.; Han, Y.; Gong, S.; Yang, L.; Hou, M. EPG Recordings reveal differential feeding behaviors in Sogatella Furcifera in response to plant virus infection and transmission success. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Backus, E.A.; Shih, H.T. Review of the epg waveforms of sharpshooters and spittlebugs including their biological meanings in relation to transmission of Xylella Fastidiosa (Xanthomonadales: Xanthomonadaceae). J. Insect Sci. 2020, 20, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Avosani, S.; Berardo, A.; Pugno, N.M.; Verrastro, V.; Mazzoni, V.; Cornara, D. Vibrational disruption of feeding behaviors of a vector of plant pathogen. Entomol. Gen. 2021, 41, 481–495. [Google Scholar] [CrossRef]
- Baric, S.; Öttl, S.; Dalla Via, J. Infection rates of natural psyllid populations with ‘Candidatus Phytoplasma mali’in South Tyrol (Northern Italy). In Proceedings of the 21st International Conference on Virus and other Graft Transmissible Diseases of Fruit Crops, Neustadt, Germany, 5–10 July 2009; Volume 427, p. 189. [Google Scholar]
- Carraro, L.; Ferrini, F.; Ermacora, P.; Loi, N.; Labonne, G. Infectivity of Cacopsylla picta (syn. Cacopsylla costalis), vector of “Candidatus Phytoplasma mali” in northeast Italy. Acta Hortic. 2008, 781, 403–408. [Google Scholar] [CrossRef]
- Jarausch, B.; Fuchs, A.; Schwind, N.; Krczal, G.; Jarausch, W. Cacopsylla picta as most important vector for ‘Candidatus Phytoplasma mali’ in Germany and neighbouring regions. Bull. Insectology 2007, 60, 189–190. [Google Scholar]
- Malagnini, V.; Pedrazzoli, F.; Gualandri, V.; Zasso, R.; Bozza, E.; Fiamingo, F.; Pozzebon, A.; Mori, N.; Ioriatti, C. Detection of “Candidatus Phytoplasma mali” in different populations of Cacopsylla Melanoneura in Italy. Bull. Insectology 2010, 63, 59–63. [Google Scholar]
- Oppedisano, T.; Panassiti, B.; Pedrazzoli, F.; Mittelberger, C.; Bianchedi, P.L.; Angeli, G.; de Cristofaro, A.; Janik, K.; Anfora, G.; Ioriatti, C. Importance of psyllids’ life stage in the epidemiology of apple proliferation phytoplasma. J. Pest Sci. 2020, 93, 49–61. [Google Scholar] [CrossRef]
- Bosco, D.; Marzachì, C. Insect transmission of phytoplasmas. In Vector-Mediated Transmission of Plant Pathogens; Brown, J.K., Ed.; The American Phytopathological Society: St. Paul, MN, USA, 2016; pp. 319–327. ISBN 978-0-89054-535-5. [Google Scholar]
- Danielli, A.; Bertaccini, A.; Vibio, M.; Mori, N.; Murari, E.; Posenato, G.; Girolami, V. Detection and molecular characterization of phytoplasmas in the planthopper Metcalfa pruinosa (Say)(Homoptera: Flatidae). Phytopathol. Mediterr. 1996, 35, 62–65. [Google Scholar]
- Tedeschi, R.; Lauterer, P.; Brusetti, L.; Tota, F.; Alma, A. Composition, abundance and phytoplasma infection in the hawthorn psyllid fauna of northwestern.n Italy. Eur. J. Plant Pathol. 2009, 123, 301–310. [Google Scholar] [CrossRef]
- Miñarro, M.; Somoano, A.; Moreno, A.; García, R.R. Candidate insect vectors of apple proliferation in Northwest Spain. SpringerPlus 2016, 5, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cainelli, C.; Forno, F.; Mattedi, L.; Grando, M.S. Can apple aphids be vectors of “Candidatus Phytoplasma mali”? In Proceedings of the International Workshop on Arthropod Pest Problems in Pome Fruit Production, Lleida, Spain, 4–6 September 2006; pp. 261–266. [Google Scholar]
- Mattedi, L.; Forno, F.; Cainelli, C.; Grando, M.S.; Jarausch, W. Research on Candidatus phytoplasma mali transmission by insect vectors in Trentino. Acta Hortic. 2008, 781, 369–374. [Google Scholar] [CrossRef]
- Bosco, D.; Tedeschi, R. Insect Vector Transmission Assays. In Phytoplasma. Methods in Molecular Biology; Dickinson, M., Hodgetts, J., Eds.; Humana Press: Totowa, NJ, USA, 2013; Volume 938, pp. 73–85. [Google Scholar]






| Treatments | Estimate | Std. Error | z Value | Pr (>|z|) |
|---|---|---|---|---|
| Treatment 72 h | 0.157 | 0.215 | 0.729 | 0.466 |
| Treatment N h | 0.265 | 0.231 | 1.151 | 0.250 |
| Week | 0.048 | 0.045 | 1.074 | 0.283 |
| Treatment 72 h: Week | 0.082 | 0.066 | 1.241 | 0.215 |
| Treatment N h: Week | 0.288 | 0.075 | 3.826 | >0.001 |
| Intercept | −0.769 | 0.149 | −5.147 | >0.001 |
| Site | De Bellat | Mollaro |
|---|---|---|
| Data of collection | 14 August 2020 | 3 September 2020 |
| Collected MLH | 43 | 76 |
| AP-positive MLH | 8 | 12 |
| % AP-positive MLH | 18.6 | 15.7 |
| % Infected plants | 2.8 | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalmaso, G.; Ioriatti, C.; Gualandri, V.; Zapponi, L.; Mazzoni, V.; Mori, N.; Baldessari, M. Orientus ishidae (Hemiptera: Cicadellidae): Biology, Direct Damage and Preliminary Studies on Apple Proliferation Infection in Apple Orchard. Insects 2023, 14, 246. https://doi.org/10.3390/insects14030246
Dalmaso G, Ioriatti C, Gualandri V, Zapponi L, Mazzoni V, Mori N, Baldessari M. Orientus ishidae (Hemiptera: Cicadellidae): Biology, Direct Damage and Preliminary Studies on Apple Proliferation Infection in Apple Orchard. Insects. 2023; 14(3):246. https://doi.org/10.3390/insects14030246
Chicago/Turabian StyleDalmaso, Giovanni, Claudio Ioriatti, Valeria Gualandri, Livia Zapponi, Valerio Mazzoni, Nicola Mori, and Mario Baldessari. 2023. "Orientus ishidae (Hemiptera: Cicadellidae): Biology, Direct Damage and Preliminary Studies on Apple Proliferation Infection in Apple Orchard" Insects 14, no. 3: 246. https://doi.org/10.3390/insects14030246
APA StyleDalmaso, G., Ioriatti, C., Gualandri, V., Zapponi, L., Mazzoni, V., Mori, N., & Baldessari, M. (2023). Orientus ishidae (Hemiptera: Cicadellidae): Biology, Direct Damage and Preliminary Studies on Apple Proliferation Infection in Apple Orchard. Insects, 14(3), 246. https://doi.org/10.3390/insects14030246