Applying Satyrization to Insect Pest Control: The Case of the Spotted Wing Drosophila, Drosophila suzukii Matsumura
Abstract
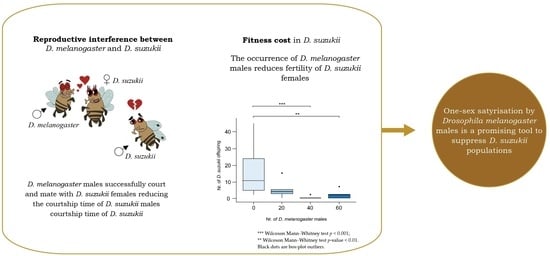
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment 1: Analysis of Courtship Behavior between D. suzukii and D. melanogaster
2.2. Experiment 2: Analysis of Insemination between D. suzukii and D. melanogaster
2.3. Experiment 3: Analysis of Larval Development Resulting from Insemination by D. melanogaster
2.4. Experiment 4: Analysis of Satyrization of D. melanogaster Males on the Fertility of D. suzukii
2.5. Data Analysis
3. Results
3.1. Experiment 1: Courtship Behavior between D. suzukii and D. melanogaster
3.2. Experiment 2: Insemination between D. suzukii and D. melanogaster
3.3. Experiment 3: Larval Development after Insemination by D. melanogaster
3.4. Experiment 4: Effect of Satyrization of D. melanogaster Males on the Fertility of D. suzukii
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribeiro, J.M.C. Can Satyrs Control Pests and Vectors? J. Med. Entomol. 1988, 25, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Gröning, J.; Hochkirch, A. Reproductive Interference Between Animal Species. Q. Rev. Biol. 2008, 83, 257–282. [Google Scholar] [CrossRef] [PubMed]
- Shuker, D.M.; Burdfield-Steel, E.R. Reproductive interference in insects: Reproductive interference in insects. Ecol. Entomol. 2017, 42, 65–75. [Google Scholar] [CrossRef]
- Kyogoku, D. When does reproductive interference occur? Predictions and data. Popul. Ecol. 2020, 62, 196–206. [Google Scholar] [CrossRef]
- Mitchell, C.; Leigh, S.; Alphey, L.; Haerty, W.; Chapman, T. Reproductive interference and Satyrisation: Mechanisms, outcomes and potential use for insect control. J. Pest Sci. 2022, 95, 1023–1036. [Google Scholar] [CrossRef]
- Kishi, S.; Nishida, T.; Tsubaki, Y. Reproductive interference determines persistence and exclusion in species interactions: Sexual interference governs competition. J. Anim. Ecol. 2009, 78, 1043–1049. [Google Scholar] [CrossRef]
- Serrano, J.M.; Castro, L.; Toro, M.A.; López-Fanjul, C. Inter- and intraspecific sexual discrimination in the flour beetles Tribolium castaneum and Tribolium confusum. Heredity 2000, 85, 142–146. [Google Scholar] [CrossRef]
- Kishi, S. Reproductive interference in laboratory experiments of interspecific competition. Popul. Ecol. 2015, 57, 283–292. [Google Scholar] [CrossRef]
- Liu, S.-S.; De Barro, P.J.; Xu, J.; Luan, J.-B.; Zang, L.-S.; Ruan, Y.-M.; Wan, F.-H. Asymmetric Mating Interactions Drive Widespread Invasion and Displacement in a Whitefly. Science 2007, 318, 1769–1772. [Google Scholar] [CrossRef]
- Miller, J.R.; Spencer, J.L.; Lentz, A.J.; Keller, J.E.; Walker, E.D.; Leykam, J.F. Sex peptides: Potentially important and useful regulators of insect reproduction. In Natural and Engineered Pest Management Agents; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1994; Volume 551, pp. 189–209. [Google Scholar]
- Honma, A.; Kumano, N.; Noriyuki, S. Killing two bugs with one stone: A perspective for targeting multiple pest species by incorporating reproductive interference into sterile insect technique. Pest Manag. Sci. 2019, 75, 571–577. [Google Scholar] [CrossRef]
- Alphey, L.; McKemey, A.; Nimmo, D.; Neira Oviedo, M.; Lacroix, R.; Matzen, K.; Beech, C. Genetic control of Aedes mosquitoes. Pathog. Glob. Health 2013, 107, 170–179. [Google Scholar] [CrossRef]
- Alphey, L. Genetic Control of Mosquitoes. Annu. Rev. Èntomol. 2014, 59, 205–224. [Google Scholar] [CrossRef]
- Tait, G.; Mermer, S.; Stockton, D.; Lee, J.; Avosani, S.; Abrieux, A.; Anfora, G.; Beers, E.; Biondi, A.; Burrack, H.; et al. Drosophila suzukii (Diptera: Drosophilidae): A Decade of Research Towards a Sustainable Integrated Pest Management Program. J. Econ. Èntomol. 2021, 114, 1950–1974. [Google Scholar] [CrossRef]
- Sasaki, M.; Sato, R. Bionomics of the cherry drosophila, Drosophila suzukii Matsumura (Diptera: Drosophilidae) in Fukushima prefecture [Japan], 2: Overwintering and number of generations. Annu. Rep. Soc. Plant Prot. N. Jpn. 1995, 46, 167–169. (In Japanese) [Google Scholar]
- Cini, A.; Anfora, G.; Escudero-Colomar, L.A.; Grassi, A.; Santosuosso, U.; Seljak, G.; Papini, A. Tracking the invasion of the alien fruit pest Drosophila suzukii in Europe. J. Pest Sci. 2014, 87, 559–566. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Hu, W.; Li, J.; Liu, P.; Hu, H.Y. The mating rate of Drosophila suzukii reduction due to reproductive interference from Drosophila melanogaster. Res. Sq. 2020, preprint. [Google Scholar] [CrossRef]
- Tran, A.K.; Hutchison, W.D.; Asplen, M.K. Morphometric criteria to differentiate Drosophila suzukii (Diptera: Drosophilidae) seasonal morphs. PLoS ONE 2020, 15, e0228780. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Lasbleiz, C.; Ferveur, J.-F.; Everaerts, C. Courtship behaviour of Drosophila melanogaster revisited. Anim. Behav. 2006, 72, 1001–1012. [Google Scholar] [CrossRef]
- Revadi, S.; Lebreton, S.; Witzgall, P.; Anfora, G.; Dekker, T.; Becher, P.G. Sexual Behavior of Drosophila suzukii. Insects 2015, 6, 183–196. [Google Scholar] [CrossRef]
- Avanesyan, A.; Jaffe, B.D.; Guédot, C. Isolating Spermathecae and Determining Mating Status of Drosophila suzukii: A Protocol for Tissue Dissection and Its Applications. Insects 2017, 8, 32. [Google Scholar] [CrossRef]
- Friard, O.; Gamba, M. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Schetelig, M.F.; Lee, K.-Z.; Otto, S.; Talmann, L.; Stökl, J.; Degenkolb, T.; Vilcinskas, A.; Halitschke, R. Environmentally sustainable pest control options forDrosophila suzukii. J. Appl. Èntomol. 2018, 142, 3–17. [Google Scholar] [CrossRef]
- Haudry, A.; Laurent, S.; Kapun, M. Population Genomics on the Fly: Recent Advances in Drosophila. Methods Mol. Biol. 2020, 2090, 357–396. [Google Scholar] [CrossRef]
- Markow, T. The secret lives of Drosophila flies. eLife 2015, 4, e06793. [Google Scholar] [CrossRef] [PubMed]
- Dekker, T.; Revadi, S.; Mansourian, S.; Ramasamy, S.; Lebreton, S.; Becher, P.G.; Angeli, S.; Rota-Stabelli, O.; Anfora, G. Loss of Drosophila pheromone reverses its role in sexual communication in Drosophila suzukii. Proc. R. Soc. B Boil. Sci. 2015, B282, 20143018. [Google Scholar] [CrossRef] [PubMed]
- Shaw, B.; Brain, P.; Wijnen, H.; Fountain, M.T. Reducing Drosophila suzukii emergence through inter-species competition. Pest Manag. Sci. 2018, 74, 1466–1471. [Google Scholar] [CrossRef]
- Kyogoku, D.; Sota, T. A generalized population dynamics model for reproductive interference with absolute density dependence. Sci. Rep. 2017, 7, 1996. [Google Scholar] [CrossRef]
- Fowler, K.; Partridge, L. A cost of mating in female fruit flies. Nature 1989, 338, 760–761. [Google Scholar] [CrossRef]
- Chapman, T.; Liddle, L.F.; Kalb, J.M.; Wolfner, M.F.; Partridge, L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 1995, 373, 241–244. [Google Scholar] [CrossRef]
- Liou, L.W.; Price, T.D. Speciation by reinforcement of premating isolation. Evolution 1994, 48, 1451–1459. [Google Scholar] [CrossRef]
- Kyogoku, D. Reproductive interference: Ecological and evolutionary consequences of interspecific promiscuity. Popul. Ecol. 2015, 57, 253–260. [Google Scholar] [CrossRef]
- Servedio, M.R.; Noor, M.A.F. The Role of Reinforcement in Speciation: Theory and Data. Ann. Rev. Ecol. Evol. System. 2003, 34, 339–364. [Google Scholar] [CrossRef]
- Matute, D.R. Reinforcement of Gametic Isolation in Drosophila. PLoS Biol. 2010, 8, e1000341. [Google Scholar] [CrossRef] [PubMed]
- Urbanelli, S.; Porretta, D.; Mastrantonio, V.; Bellini, R.; Pieraccini, G.; Romoli, R.; Crasta, G.; Nascetti, G. Data from: Hybridization, natural selection and evolution of reproductive isolation: A 25-years survey of an artificial sympatric area between two mosquito sibling species of the Aedes mariae complex. Evolution 2014, 68, 3030–3038. [Google Scholar] [CrossRef] [PubMed]
- Dyck, V.A.; Hendrichs, J.; Robinson, A.S. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Taylor & Francis: Boca Raton, FL, USA, 2021. [Google Scholar]
- Homem, R.A.; Mateos-Fierro, Z.; Jones, R.; Gilbert, D.; Mckemey, A.R.; Slade, G.; Fountain, M.T. Field Suppression of Spotted Wing Drosophila (SWD) (Drosophila suzukii Matsumura) Using the Sterile Insect Technique (SIT). Insects 2022, 13, 328. [Google Scholar] [CrossRef] [PubMed]
- Noor, M.A. Speciation driven by natural selection in Drosophila. Nature 1995, 375, 674–675. [Google Scholar] [CrossRef] [PubMed]
- Ardeh, M.J.; de Jong, P.W.; Loomans, A.J.M.; van Lenteren, J.C. Inter- and Intraspecific Effects of Volatile and Nonvolatile Sex Pheromones on Males, Mating Behavior, and Hybridization in Eretmocerus mundus and E. eremicus (Hymenoptera: Aphelinidae). J. Insect Behav. 2004, 17, 745–759. [Google Scholar] [CrossRef]
- Garcia, F.R.M. Introduction to Drosophila suzukii Management; Springer Nature: Basel, Switzerland, 2020; pp. 1–9. [Google Scholar] [CrossRef]
- Lanouette, G.; Brodeur, J.; Fournier, F.; Martel, V.; Vreysen, M.; Cáceres, C.; Firlej, A. The sterile insect technique for the management of the spotted wing drosophila, Drosophila suzukii: Establishing the optimum irradiation dose. PLoS ONE 2017, 12, e0180821. [Google Scholar] [CrossRef]
- Sassù, F.; Nikolouli, K.; Caravantes, S.; Taret, G.; Pereira, R.; Vreysen, M.J.B.; Stauffer, C.; Cáceres, C. Mass-Rearing of Drosophila suzukii for Sterile Insect Technique Application: Evaluation of Two Oviposition Systems. Insects 2019, 10, 448. [Google Scholar] [CrossRef] [PubMed]
- Gleason, J.M.; Roy, P.R.; Everman, E.R.; Gleason, T.C.; Morgan, T.J. Phenology of Drosophila species across a temperate growing season and implications for behavior. PLoS ONE 2019, 14, e0216601. [Google Scholar] [CrossRef] [PubMed]
- Clymans, R.; Van Kerckvoorde, V.; Thys, T.; De Clercq, P.; Bylemans, D.; Beliën, T. Mass Trapping Drosophila suzukii, What Would It Take? A Two-Year Field Study on Trap Interference. Insects 2022, 13, 240. [Google Scholar] [CrossRef] [PubMed]
- Rundle, H.D.; Schluter, D. Natural selection and ecological speciation in sticklebacks. In Adaptive Speciation; Dieckmann, U., Doebeli, M., Metz, J.A.J., Tautz, D., Eds.; Cambridge Univ. Press: Cambridge, UK, 2004; pp. 192–209. [Google Scholar]
- Jennings, J.H.; Etges, W.J. Species hybrids in the laboratory but not in nature: A reanalysis of premating isolation between Drosophila arizonae and D. mojavensis. Evolution 2010, 64, 587–598. [Google Scholar] [CrossRef] [PubMed]


| Courtship Elements | Courtship Behavior of D. melanogaster Males | Courtship Behavior of D. suzukii Males | ||
|---|---|---|---|---|
| 1 ♂D. mel. + 1 ♀ D. suz. | 1 ♂D. mel. + 1 ♀ D. suz. + 1 ♂D. suz | 1♂ D. suz. + 1♀ D. suz. | 1♂ D. suz. + 1 ♀ D. suz. + 1♂ D. mel. | |
| Orientation | 1.28 (±0.44) | 0.74 (±0.20) | 0.03 (±0.02) | 0.41 (±0.15) |
| Tapping | 3.14 (±0.72) | 1.60 + (±0.45) | 4.20 (±2.32) | 0.77 (±0.31) |
| Wing spreading | 13.42 (±2.40) | 8.46 (±2.54) | 20.82 (±3.26) | 5.70 (±1.56) |
| Wing scissoring | 0.29 (±0.12) | 0.15 (±0.06) | 0.52 (±0.52) | 0.09 (±0.05) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerasti, F.; Mastrantonio, V.; Dallai, R.; Cristofaro, M.; Porretta, D. Applying Satyrization to Insect Pest Control: The Case of the Spotted Wing Drosophila, Drosophila suzukii Matsumura. Insects 2023, 14, 569. https://doi.org/10.3390/insects14060569
Cerasti F, Mastrantonio V, Dallai R, Cristofaro M, Porretta D. Applying Satyrization to Insect Pest Control: The Case of the Spotted Wing Drosophila, Drosophila suzukii Matsumura. Insects. 2023; 14(6):569. https://doi.org/10.3390/insects14060569
Chicago/Turabian StyleCerasti, Flavia, Valentina Mastrantonio, Romano Dallai, Massimo Cristofaro, and Daniele Porretta. 2023. "Applying Satyrization to Insect Pest Control: The Case of the Spotted Wing Drosophila, Drosophila suzukii Matsumura" Insects 14, no. 6: 569. https://doi.org/10.3390/insects14060569
APA StyleCerasti, F., Mastrantonio, V., Dallai, R., Cristofaro, M., & Porretta, D. (2023). Applying Satyrization to Insect Pest Control: The Case of the Spotted Wing Drosophila, Drosophila suzukii Matsumura. Insects, 14(6), 569. https://doi.org/10.3390/insects14060569