Integrated Identification and Genetic Diversity of Potentially Invasive Clearwing Moths (Lepidoptera: Cossoidea: Sesiidae) in Korea
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling from the Field
2.2. Molecular Protocol
2.3. Data Analysis
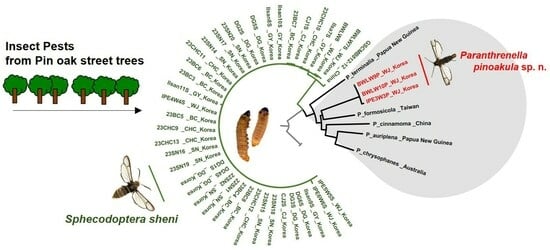
3. Results
3.1. Morphological Identification
Taxonomic Accounts
- Genus Sphecodoptera Hampson, [1893] [27].
- Sphecia; Hampson, 1919 [28]: 80.
- Sesia; Spatenka, Lastuvka, Gorbunov, Tosevski, and Arita, 1993 [29]: 87.
- Spherodoptera; Matsumura, 1931 [11]: 1017.
- Scasiba; Matsumura, 1931 [30]: 8.
- Sphecodoptera sheni (Arita and Xu, 1994) [30]
- Sesia sheni; Arita and Xu, 1994 [30]: 61. Type locality. Nanjing, Jiangsu, China.
- Scasiba caryavora; Xu and Arita, 1994 [31]: 2.
- Sphecodoptera sheni; Kallies, Arita, Owada. and Wang, 2014 [32]: 587.
- Genus Paranthrenella Strand, 1916 [36].
- Paranthrenella pinoakula sp. nov. Kim
3.2. Molecular Analyses
Genetic Divergence and Distribution
3.3. NJ Tree Analysis
3.4. Haplotype Analysis
3.5. Phylogenetic Tree
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boisduval, J.A. Europaeorum Lepidopterorum Index Methodicus, Pars Prima, Sistens Genera Papilio, Sphinx, Bombyx et Noctua Lin; Méquignon-Marvis: Paris, France, 1828; Volume 1, p. 103. [Google Scholar]
- Kallies, A. The clearwing moths (Lepidoptera, Sesiidae) of Australia, New Guinea and the Pacific Islands. Zootaxa 2020, 4833, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Pühringer, F.; Kallies, A. Checklist of the Sesiidae of the World (Lepidoptera: Ditrysia). (Latest Update: 2-January-2024). Available online: https://www.sesiidae.net/Checklst.htm (accessed on 10 January 2024).
- Lâstůvka, Z.; Lâstůvka, A. The Sesiidae of Europe; Apollo Books: Stenstrup, Denmark, 2001; p. 245. [Google Scholar]
- Arita, Y.; Bae, Y.S.; Lee, C.M.; Ikeda, M. Sesiidae (Lepidoptera) of Korea. Trans. Lepidopterol. Soc. Jpn. 2004, 55, 1–12. [Google Scholar]
- Lee, C.M.; Arita, Y.; Bae, Y.-S. A Newly Recorded Species of the Genus Milisipepsis (Lepidoptera, Sesiidae) from Korea. Korean J. Appl. Entomol. 2011, 50, 363–365. [Google Scholar] [CrossRef]
- Lee, C.M.; Bae, Y.S.; Arita, Y. Morphological description of Synanthedon bicingulata (Staudinger, 1887) in life stages (Lepidoptera, Sesiidae). J. Asia-Pac. Entomol. 2004, 7, 177–185. [Google Scholar] [CrossRef]
- Lee, C.M.; Arita, Y.; Bae, Y.S. Taxonomic study of the adult and immature stages of the clearwing moth, Synanthedon haitangvora Yang (Lepidoptera, Sesiidae), injurious to apple trees in Korea. Trans. Lepidopterol. Soc. Jpn. 2005, 56, 51–60. [Google Scholar]
- Lee, K.C.; Park, C.G. Seasonal occurrence of smaller clearwing moth, Synanthedon tenuis in sweet persimmon orchards. Korean J. Appl. Entomol. 2003, 42, 165–167. [Google Scholar]
- Lee, S.; Kim, C.; Lee, K.H.; Lee, J.W.; Oh, H.K.; Han, J.; Kim, S.H.; Kim, Y. Seasonal Occurrence of Three Pest Moths in Jujube Orchards in Boeun, Korea. Korean J. Appl. Entomol. 2017, 56, 261–265. [Google Scholar] [CrossRef]
- Matsumura, S. 6000 Illustrated Insect of Japan; The Toko-shoin: Tokyo, Japan, 1931; pp. 1012–1109. [Google Scholar]
- Matsumura, S. A list and new species of Aegeridae from Japan. Insecta Matsumurana 1931, 6, 4–12. [Google Scholar]
- Park, J.K.; Lee, J.E. Check List of Insects from Korea; Korean Society of Applied Entomology & The Entomological Society of Korea, Paper and Pencil: Daegu, Republic of Korea, 2021; p. 1055. [Google Scholar]
- Arita, Y. Sesiidae. In The Standard of Moths in Japan III; Hirowatari, T., Nasu, Y., Sakamaki, Y., Kishida, Y., Eds.; Gakken Education Publishing: Tokyo, Japan, 2013; pp. 332–341. [Google Scholar]
- Yagi, S.; Hirowatari, T.; Arita, Y. A remarkable new species of the genus Teinotarsina (Lepidoptera, Sesiidae) from Okinawa-jima, Japan. ZooKeys 2016, 571, 143–152. [Google Scholar]
- Jin, Q.; Wnag, S.X.; Li, H.H. Catalogue of the family Sesiidae in China (Lepidoptera: Sesiidae). Shilap-Rev. De Lepidopterol. 2008, 36, 507–526. [Google Scholar]
- Kim, J.K.; Koh, S.H.; Goo, C.D.; Kim, K.W.; Kim, J.G.; Kim, J.J.; Park, G.S.; Park, Y.C.; Park, I.K.; Byun, B.K.; et al. Forest Protection; Haengmoonsa: Seoul, Republic of Korea, 2019; p. 430. [Google Scholar]
- Kim, S.; Lee, Y.; Mutanen, M.; Seung, J.; Lee, S. high functionality of DNA barcodes and revealed cases of cryptic diversity in Korean curved-horn moths (Lepidoptera: Gelechioidea). Sci. Rep. 2020, 10, 6208. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, W.; Lee, S. Estimation of a new molecular marker of the genus Stathmopoda (Lepidoptera: Stathmopodidae): Comparing EF1a and COI sequences. J. Asia-Pac. Entomol. 2017, 20, 269–280. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxi dase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Molecu Larbiology Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmid, H.A.; Chernomor, O.; Dominik, S.; Michael, D.W.; Arndt, V.H.; Robert, L. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Chernomor, O.; Haeseler, A.; Minh, B.Q. Terrace aware data structure for phylogenomic inference from supermatrices. Syst. Biol. 2016, 65, 997–1008. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Anisimova, M.; Gil, M.; Dufayard, J.F.; Dessimoz, C.; Gascuel, O. Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes. Syst. Biol. 2011, 60, 685–699. [Google Scholar] [CrossRef]
- Hampson, G.F. The Fauna of British India, Including Geylon and Burma. Moths. Vol. I.; Taylor and Francis, Red Lion Court, Fleet Street: London, UK, 1893; pp. 1–527. [Google Scholar]
- Hampson, G.F. A classification of the Aegeriadae of the Oriental and Ethiopian regions. Novit. Zool. 1919, 26, 46–119. [Google Scholar] [CrossRef]
- Spatenka, K.; Lastuvka, Z.; Gorbunov, O.; Tosevski, I.; Arita, Y. Dis Systematik und Synonymie der paläarktischen Glasflügler-Arten (Leidoptera, Sesiidae). Nachrichten Entomol. Ver. Apollo 1993, 14, 81–114. [Google Scholar]
- Arita, Y.; Xu, Z.-G.; Liu, Y.-Q. A new Sesia (Lepidoptera, Sesiidae), clearwing borer on Pecan from Nanjing, China. Tinea 1994, 14, 61–64. [Google Scholar]
- Xu, Z.G.; Jin, T.; Liu, X.L. A summary and a new species of the genus Scasiba (Lepidoptera, Sesiidae). Acta Agri Cult. Boreali-Occident. Sin. 1994, 3, 1–5. [Google Scholar]
- Kallies, A.; Arita, Y.; Owada, M.; Wang, M. laetosphecia, a new genus of clearwing moths from south-eastern China, with a brief review of the Sesiini from China (Lepidoptera, Sesiidae). Zootaxa 2014, 3895, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Arita, Y.; Fukuzumi, K. Description of the Male of Sesia rhynchioides (Butler) (Lepidoptera, Sesiidae). Tyô Ga 1988, 39, 187–191. [Google Scholar]
- Lee, C.M. Systematic Study of the Family Sesiidae (Insecta, Lepidoptera) from Korea; University of Incheon: Incheon, Republic of Korea, 2003; p. 141. [Google Scholar]
- Shen, B.Y. Primary study of Aegeria molybdoceps Hampson. Plant Prot. 1988, 3, 23–25. [Google Scholar]
- Strand, E.H. Sauter’s Formosa-Ausbeute: Noctuidae p.p. (Agaristinae, Macrobrochis), Aganaidae, Saturniidae, Uraniidae, Cossidae, Callidulidae und Aegeriidae. Arch. Naturgeschichte 1916, 81, 34–49. [Google Scholar]
- Yu, T.; Gao, L.; Kallies, A.; Arita, Y.; Wang, M. A new species of the genus Paranthrenella Strand, 1916 (Lepidoptera: Sesiidae) from China. Zootaxa 2021, 4920, 123–130. [Google Scholar] [CrossRef]
- Lehmann, P.; Ammunét, T.; Barton, M.; Battisti, A.; Eigenbrode, S.D.; Jepsen, J.U.; Kalinkat, G.; Neuvonen, S.; Niemelä, P.; Terblanche, J.S.; et al. Complex responses of global insect pests to climate warming. Front. Ecol. Environ. 2020, 18, 141–150. [Google Scholar] [CrossRef]
- World Economic Forum. Global Risks Report 2023, 18th ed.; Insight Report; World Economic forum: Geneva, Switzerland, 2023; p. 98. [Google Scholar]
- Lee, W.; Lee, Y.; Kim, S.; Lee, J.-H.; Lee, H.; Lee, S.; Hong, K.-J. Current status of exotic insect pests in Korea: Comparing border interception and incursion during 1996–2014. J. Asia-Pac. Entomol. 2016, 19, 1095–1101. [Google Scholar] [CrossRef]










| (1) Different taxonomic levels | ||||
| Taxonomic level (No. of Comparison Pairs) | K2P Pairwase distances | |||
| Maximum | Minimum | Average | ||
| Within species (647) | 1.9 | 0 | 0.3 | |
| Between species (564) | 16.8 | 8.1 | 13.8 | |
| (2) Intraspecific genetic divergence | ||||
| Species | Comparison pairs (CP) | Intraspecific genetic divergence | ||
| Maximum | Minimum | Average | ||
| S. sheni | 637 | 1.7 | 0 | 0.2 |
| P. pinoakula sp.n. | 3 | 0.3 | 0 | 0.2 |
| P. auriplena | - | - | - | - |
| P. cinnamoma | - | - | - | - |
| P. chrysophanes | 6 | 1.9 | 0 | 0.9 |
| P. formosicola | - | - | - | - |
| P. terminalis | - | - | - | - |
| A. japonica | 1 | 0 | 0 | 0 |
| (3) Interspecific genetic divergence | ||||
| species | S. sheni | P. pinoakula sp. n. | P. auriplena | P. cinnamoma |
| S. sheni | CP = 114, 14.6 (14.2–15.3) | CP = 38, 14.2 (13.9–14.9) | CP = 38, 15.1 (14.9–15.8) | |
| P. pinoakula sp.n. | CP = 3, 13.9 (13.9–13.9) | CP = 3, 13.0 (13.0–13.0) | ||
| P. auriplena | CP = 1, 11.5 (11.5–11.5) | |||
| P. cinnamoma | ||||
| P. chrysophanes | ||||
| P. formosicola | ||||
| P. terminalis | ||||
| A. japonica | CP = 78, 11.8 (11.5–12.5) | CP = 6, 14.9 (14.9–14.9) | CP = 2, 13.7 (13.7–13.7) | CP = 2, 15.6 (15.6–15.6) |
| species | P. chrysophanes | P. formosicola | P. terminalis | |
| S. sheni | CP = 152, 14.0 (13.7–14.9) | CP = 38, 13.1 (12.9–13.7) | CP = 38, 13.7 (13.4–14.4) | |
| P. pinoakula sp.n. | CP = 12, 13.4 (13.0–13.7) | CP = 3, 11.8 (11.7–12.0) | CP = 3, 14.3 (14.2–14.4) | |
| P. auriplena | CP = 4, 8.2 (8.1–8.4) | CP = 1, 13.2 (13.2–13.2) | CP = 1, 14.9 (14.9–14.9) | |
| P. cinnamoma | CP = 4, 138 (13.7–13.9) | CP = 1 12.2 (12.2–12.2) | CP = 1, 14.4 (14.4–14.4) | |
| P. chrysophanes | CP = 4, 14.4 (14.2–14.6) | CP = 4, 16.5 (16.3–16.8) | ||
| P. formosicola | CP = 1, 12.2 (12.2–12.2) | |||
| P. terminalis | ||||
| A. japonica | CP = 8, 15.6 (15.3–16.0) | CP = 2, 14.9 (14.9–14.9) | CP = 2, 14.1 (14.1–14.1) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Jung, J.-K.; Park, I.; Lee, B.-W.; Kim, Y.-H. Integrated Identification and Genetic Diversity of Potentially Invasive Clearwing Moths (Lepidoptera: Cossoidea: Sesiidae) in Korea. Insects 2024, 15, 79. https://doi.org/10.3390/insects15010079
Kim S, Jung J-K, Park I, Lee B-W, Kim Y-H. Integrated Identification and Genetic Diversity of Potentially Invasive Clearwing Moths (Lepidoptera: Cossoidea: Sesiidae) in Korea. Insects. 2024; 15(1):79. https://doi.org/10.3390/insects15010079
Chicago/Turabian StyleKim, Sora, Jong-Kook Jung, Ikju Park, Bong-Woo Lee, and Yong-Hun Kim. 2024. "Integrated Identification and Genetic Diversity of Potentially Invasive Clearwing Moths (Lepidoptera: Cossoidea: Sesiidae) in Korea" Insects 15, no. 1: 79. https://doi.org/10.3390/insects15010079
APA StyleKim, S., Jung, J. -K., Park, I., Lee, B. -W., & Kim, Y. -H. (2024). Integrated Identification and Genetic Diversity of Potentially Invasive Clearwing Moths (Lepidoptera: Cossoidea: Sesiidae) in Korea. Insects, 15(1), 79. https://doi.org/10.3390/insects15010079