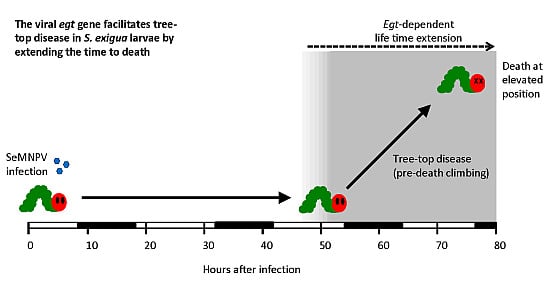
Parasitic Manipulation of Host Behaviour: Baculovirus SeMNPV EGT Facilitates Tree-Top Disease in Spodoptera exigua Larvae by Extending the Time to Death
Abstract
:1. Introduction
2. Experimental Section
2.1. Insect Larvae
2.2. SeMNPV Virus Strains and Generation of Recombinant Bacmids

2.3. Generation, Amplification and Purification of Viruses
2.4. Infectivity Assays
2.5. Mortality Assays
2.6. Behavioural Assays to Measure Tree-Top Disease
2.7. RNA Extraction and RT-PCR
3. Results
3.1. Viral Infectivity of the Generated Mutants
3.2. The SeMNPV egt Gene Extends the Time to Death of S. exigua Larvae
3.3. Deletion of the egt Gene Prevents SeMNPV-Induced Tree-Top Disease

3.4. The EGT Protein but not the egt Transcript Is Involved in This Process

3.5. The Egt Gene Is Expressed in WT and Repair Viruses

4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thomas, F.; Schmidt-Rhaesa, A.; Martin, G.; Manu, C.; Durand, P.; Renaud, F. Do hairworms (Nematomorpha) manipulate the water seeking behaviour of their terrestrial hosts? J. Evol. Biol. 2002, 15, 356–361. [Google Scholar] [CrossRef]
- Kaiser, M.; Libersat, F. The role of the cerebral ganglia in the venom-induced behavioral manipulation of cockroaches stung by the parasitoid jewel wasp. J. Exp. Biol. 2015, 218, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Van Houte, S.; Ros, V.I.D.; van Oers, M.M. Walking with insects: Molecular mechanisms behind parasitic manipulation of host behaviour. Mol. Ecol. 2013, 22, 3458–3475. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D. Pathways to understanding the extended phenotype of parasites in their hosts. J. Exp. Biol. 2013, 216, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Biron, D.G.; Loxdale, H.D. Host-parasite molecular cross-talk during the manipulative process of a host by its parasite. J. Exp. Biol. 2013, 216, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Van Houte, S.; Ros, V.I.D.; Mastenbroek, T.G.; Vendrig, N.J.; Hoover, K.; Spitzen, J.; van Oers, M.M. Protein tyrosine phosphatase-induced hyperactivity is a conserved strategy of a subset of baculoviruses to manipulate lepidopteran host behavior. PLoS ONE 2012, 7, e46933. [Google Scholar] [CrossRef] [PubMed]
- Kamita, S.G.; Nagasaka, K.; Chua, J.W.; Shimada, T.; Mita, K.; Kobayashi, M.; Maeda, S.; Hammock, B.D. A baculovirus-encoded protein tyrosine phosphatase gene induces enhanced locomotory activity in a lepidopteran host. Proc. Natl. Acad. Sci. USA 2005, 102, 2584–2589. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D. Wipfelkrankheit: Modification of host behaviour during baculoviral infection. Oecologia 1997, 109, 219–228. [Google Scholar] [CrossRef]
- Hoover, K.; Grove, M.; Gardner, M.; Hughes, D.P.; McNeil, J.; Slavicek, J. A gene for an extended phenotype. Science 2011, 333. [Google Scholar] [CrossRef] [PubMed]
- Ros, V.I.D.; van Houte, S.; Hemerik, L.; van Oers, M.M. Baculovirus-induced tree-top disease: How extended is the role of egt as a gene for the extended phenotype? Mol. Ecol. 2015, 24, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Van Houte, S.; van Oers, M.M.; Han, Y.; Vlak, J.M.; Ros, V.I.D. Baculovirus infection triggers a positive phototactic response in caterpillars to induce “tree-top” disease. Biol. Lett. 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, S.D.; Cory, J.S.; Wilson, K.R.; Sait, S.M.; Hails, R.S. Modified behavior in baculovirus-infected lepidopteran larvae and its impact on the spatial distribution of inoculum. Biol. Control 1996, 7, 299–306. [Google Scholar] [CrossRef]
- Katsuma, S.; Shimada, T. The killing speed of egt-inactivated Bombyx mori nucleopolyhedrovirus depends on the developmental stage of B. mori larvae. J. Invertebr. Pathol. 2015, 126, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Van Houte, S.; Ros, V.I.D.; van Oers, M.M. Hyperactivity and tree-top disease induced by the baculovirus AcMNPV in Spodoptera exigua larvae are governed by independent mechanisms. Naturwissenschaften 2014, 101, 347–350. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, D.R. Baculovirus-encoded ecdysteroid UDP-glucosyltransferases. Insect Biochem. Mol. Biol. 1995, 25, 541–550. [Google Scholar] [CrossRef]
- O’Reilly, D.R.; Miller, L.K. A baculovirus blocks insect molting by producing ecdysteroid UDP-glucosyl transferase. Science 1989, 245, 1110–1112. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, D.R.; Miller, L.K. Improvement of a baculovirus pesticide by deletion of the egt gene. Nature 1991, 9, 1086–1089. [Google Scholar] [CrossRef]
- Cory, J.S.; Clarke, E.E.; Brown, M.L.; Hails, R.S.; O’Reilly, D.R. Microparasite manipulation of an insect: The influence of the egt gene on the interaction between a baculovirus and its lepidopteran host. Funct. Ecol. 2004, 18, 443–450. [Google Scholar] [CrossRef]
- Bianchi, F.; Snoeijing, I.; van der Werf, W.; Mans, R.M.W.; Smits, P.H.; Vlak, J.M. Biological activity of SeMNPV, AcMNPV, and three AcMNPV deletion mutants against Spodoptera exigua larvae (Lepidoptera: Noctuidae). J. Invertebr. Pathol. 2000, 75, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Smits, P.H.; van Velden, M.C.; van de Vrie, M.; Vlak, J.M. Feeding and dispersion of Spodoptera exigua larvae and its relevance for control with a nuclear polyhedrosis virus. Entomol. Exp. Appl. 1987, 43, 67–72. [Google Scholar] [CrossRef]
- Smits, P.; van de Vrie, M.; Vlak, J. Oviposition of beet armyworm (Lepidoptera: Noctuidae) on greenhouse crops. Environ. Entomol. 1986, 15, 1189–1191. [Google Scholar] [CrossRef]
- Murillo, R.; Elvira, S.; Muñoz, D.; Williams, T.; Caballero, P. Genetic and phenotypic variability in Spodoptera exigua nucleopolyhedrovirus isolates from greenhouse soils in southern Spain. Biol. Control 2006, 38, 157–165. [Google Scholar] [CrossRef]
- Pijlman, G.P.; Dortmans, J.C.; Vermeesch, A.M.; Yang, K.; Martens, D.E.; Goldbach, R.W.; Vlak, J.M. Pivotal role of the non-hr origin of DNA replication in the genesis of defective interfering baculoviruses. J. Virol. 2002, 76, 5605–5611. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Nonaka, H.; Tsuge, Y.; Inui, M.; Yukawa, H. New multiple-deletion method for the Corynebacterium glutamicum genome, using a mutant lox sequence. Appl. Environ. Microbiol. 2005, 71, 8472–8480. [Google Scholar] [CrossRef] [PubMed]
- Luckow, V.A.; Lee, S.; Barry, G.; Olins, P. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J. Virol. 1993, 67, 4566–4579. [Google Scholar] [PubMed]
- Airenne, K.J.; Peltomaa, E.; Hytönen, V.P.; Laitinen, O.H.; Ylä-Herttuala, S. Improved generation of recombinant baculovirus genomes in Escherichia coli. Nucl. Acids Res. 2003, 31, e101. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; van Oers, M.M.; Hu, Z.; van Lent, J.W.M.; Vlak, J.M. Baculovirus per os infectivity factors form a complex on the surface of occlusion-derived virus. J. Virol. 2010, 84, 9497–9504. [Google Scholar] [CrossRef] [PubMed]
- IJkel, W.F.; van Strien, E.A.; Heldens, J.G.; Broer, R.; Zuidema, D.; Goldbach, R.W.; Vlak, J.M. Sequence and organization of the Spodoptera exigua multicapsid nucleopolyhedrovirus genome. J. Gen. Virol. 1999, 80, 3289–3304. [Google Scholar] [PubMed]
- Simon, O.; Williams, T.; Asensio, A.C.; Ros, S.; Gaya, A.; Caballero, P.; Possee, R.D. Sf29 gene of Spodoptera frugiperda multiple nucleopolyhedrovirus is a viral factor that determines the number of virions in occlusion bodies. J. Virol 2008, 82, 7897–7904. [Google Scholar] [CrossRef] [PubMed]
- LeOra Software, PoloPlus. Probit and Logit Analysis, User’s Guide; LeOra Software Inc.: Berkely, CA, USA, 2002. [Google Scholar]
- Van Oers, M.M.; van Marwijk, M.; Kwa, M.S.G.; Vlak, J.M.; Thomas, A.A.M. Cloning and analysis of cDNAs encoding the hypusine-containing protein eIF5A of two lepidopteran insect species. Insect Mol. Biol. 1999, 8, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-C.; Shiau, C.-K.; Lin, W.C. Vir-Mir db: Prediction of viral microRNA candidate hairpins. Nucleic Acids Res. 2008, 36, D184–D189. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; van Oers, M.M.; van Houte, S.; Ros, V.I.D. Virus-induced behavioural changes in insects. In Parasites and Behavioural Changes; Mehlhorn, H., Ed.; Springer: Berlin, Germany, 2015; In press. [Google Scholar]
- Bartel, D.P. Micrornas: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Kadener, S.; Menet, J.S.; Sugino, K.; Horwich, M.D.; Weissbein, U.; Nawathean, P.; Vagin, V.V.; Zamore, P.D.; Nelson, S.B.; Rosbash, M. A role for microRNAs in the Drosophila circadian clock. Genes Dev. 2009, 23, 2179–2191. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Van Houte, S.; Drees, G.F.; Van Oers, M.M.; Ros, V.I.D. Parasitic Manipulation of Host Behaviour: Baculovirus SeMNPV EGT Facilitates Tree-Top Disease in Spodoptera exigua Larvae by Extending the Time to Death. Insects 2015, 6, 716-731. https://doi.org/10.3390/insects6030716
Han Y, Van Houte S, Drees GF, Van Oers MM, Ros VID. Parasitic Manipulation of Host Behaviour: Baculovirus SeMNPV EGT Facilitates Tree-Top Disease in Spodoptera exigua Larvae by Extending the Time to Death. Insects. 2015; 6(3):716-731. https://doi.org/10.3390/insects6030716
Chicago/Turabian StyleHan, Yue, Stineke Van Houte, Gerben F. Drees, Monique M. Van Oers, and Vera I. D. Ros. 2015. "Parasitic Manipulation of Host Behaviour: Baculovirus SeMNPV EGT Facilitates Tree-Top Disease in Spodoptera exigua Larvae by Extending the Time to Death" Insects 6, no. 3: 716-731. https://doi.org/10.3390/insects6030716
APA StyleHan, Y., Van Houte, S., Drees, G. F., Van Oers, M. M., & Ros, V. I. D. (2015). Parasitic Manipulation of Host Behaviour: Baculovirus SeMNPV EGT Facilitates Tree-Top Disease in Spodoptera exigua Larvae by Extending the Time to Death. Insects, 6(3), 716-731. https://doi.org/10.3390/insects6030716