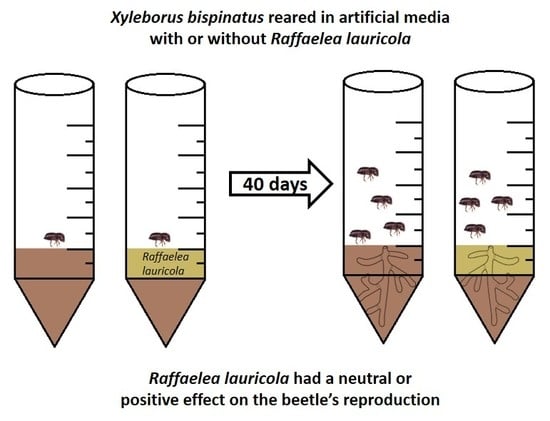
Xyleborus bispinatus Reared on Artificial Media in the Presence or Absence of the Laurel Wilt Pathogen (Raffaelea lauricola)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Artificial Media
2.2. Inoculation of Media
2.3. Rearing Conditions and Dissection of Colonies
2.4. Fungal Isolation and Identification
2.5. Data Analysis
3. Results
3.1. Medium 1
3.2. Medium 2
3.3. Medium 3
3.4. Recovery of R. lauricola and Other Fungi from X. bispinatus Reared on Artificial Media
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fraedrich, S.W.; Harrington, T.C.; Rabaglia, R.J.; Ulyshen, M.D.; Mayfield, A.E., III; Hanula, J.L.; Eickwort, J.M.; Miller, D.R. A fungal symbiont of redbay ambrosia beetle causes a lethal wilt in redbay and other Lauraceae in the southern United States. Plant Dis. 2008, 92, 215–224. [Google Scholar] [CrossRef]
- Harrington, T.C.; Fraedrich, S.W.; Aghayeva, D.N. Raffaelea lauricola, a new ambrosia beetle symbiont and pathogen on the Lauraceae. Mycotaxon 2008, 104, 399–404. [Google Scholar]
- Mayfield, A.E., III. Laurel wilt: A serious threat to redbay and other related native plants. Palmetto 2007, 24, 8–11. [Google Scholar]
- Fraedrich, S.W.; Harrington, T.C.; Bates, C.A.; Johnson, J.; Reid, L.S.; Best, G.S.; Leininger, T.D.; Hawkins, T.S. Susceptibility to laurel wilt and disease incidence in two rare plant species, pondberry and pondspice. Plant Dis. 2011, 95, 1056–1062. [Google Scholar] [CrossRef]
- Kendra, P.E.; Montgomery, W.S.; Niogret, J.; Pruett, G.E.; Mayfield, A.E., III; MacKenzie, M.; Deyrup, M.A.; Bauchan, G.R.; Ploetz, R.C.; Epsky, N.D. North American Lauraceae: Terpenoid emissions, relative attraction and boring preferences of redbay ambrosia beetle, Xyleborus glabraus (Coleoptera: Curculionidae: Scolytinae). PLoS ONE 2014, 9, e102086. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.A.; Smith, J.A.; Ploetz, R.C.; Kendra, P.E.; Mayfield, A.E., III; Hanula, J.; Hulcr, J.; Stelinski, L.L.; Cameron, S.; Riggins, J.J.; et al. Recovery plan for laurel wilt on redbay and other forest species caused by Raffaelea lauricola and disseminated by Xyleborus glabratus. Plant Health Prog. 2015, 16, 173–210. [Google Scholar] [CrossRef]
- Carrillo, D.; Duncan, R.E.; Peña, J.E. Ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) that breed in avocado wood in Florida. Fla. Entomol. 2012, 95, 573–579. [Google Scholar] [CrossRef]
- Kendra, P.E.; Owens, D.; Montgomery, W.S.; Narvaez, T.I.; Bauchan, G.R.; Schnell, E.Q.; Tabanca, N.; Carrillo, D. α-Copaene is an attractant, synergistic with quercivorol, for improved detection of Euwallacea nr. fornicatus (Coleoptera: Curculionidae: Scolytinae). PLoS ONE 2017, 12, e0179416. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, D.; Duncan, R.E.; Ploetz, J.N.; Campbell, A.F.; Ploetz, R.C.; Peña, J.E. Lateral transfer of a phytopathogenic symbiont among native and exotic ambrosia beetles. Plant Pathol. 2014, 63, 54–62. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Konkol, J.L.; Narvaez, T.; Duncan, R.E.; Saucedo, R.J.; Campbell, A.; Mantilla, J.; Carrillo, D.; Kendra, P.E. Presence and prevalence of Raffaelea lauricola, cause of laurel wilt, in different species of ambrosia beetle in Florida, USA. J. Econ. Entomol. 2017, 110, 347–354. [Google Scholar] [PubMed]
- Atkinson, T.H.; Carrillo, D.; Duncan, R.E.; Peña, J.E. Occurrence of Xyleborus bispinatus (Coleoptera: Curculionidae: Scolytinae) Eichhoff in southern Florida. Zootaxa 2013, 3669, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, T.H.; Peck, S.B. Annotated checklist of the bark and ambrosia beetles (Coleoptera: Scolytidae) of tropical southern Florida. Fla. Entomol. 1994, 77, 313–329. [Google Scholar] [CrossRef]
- Kirkendall, L.R.; Jordal, B.H. The bark and ambrosia beetles (Curculionidae, Scolytinae) of Cocos Island, Costa Rica and the role of mating systems in island zoogeography. Biol. J. Linn. Soc. 2006, 89, 729–743. [Google Scholar] [CrossRef]
- Faccoli, M.; Campo, G.; Perrotta, G.; Rassati, D. Two newly introduced tropical bark and ambrosia beetles (Coleoptera: Curculionidae, Scolytinae) damaging figs (Ficus carica) in southern Italy. Zootaxa 2016, 4136, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Rabaglia, R.J.; Dole, S.A.; Cognato, A.I. Review of American Xyleborina (Coleoptera: Curculionidae: Scolytinae) occurring North of Mexico, with an illustrated key. Ann. Entomol. Soc. Am. 2006, 99, 1034–1056. [Google Scholar] [CrossRef]
- Kendra, P.E.; Niogret, J.; Montgomery, W.S.; Deyrup, M.A.; Epsky, N.D. Cubeb oil lures: Terpenoid emissions, trapping efficacy, and longevity for attraction of redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae). J. Econ. Entomol. 2015, 108, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Kendra, P.E.; Montgomery, W.S.; Schnell, E.Q.; Deyrup, M.A.; Epsky, N.D. Efficacy of α-copaene, cubeb, and eucalyptol lures for detection of redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae). J. Econ. Entomol. 2016, 109, 2428–2435. [Google Scholar] [CrossRef] [PubMed]
- Six, D.L. Bark beetle–fungus symbioses. In Insect Symbiosis; Bourtzis, K., Miller, T.A., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 97–114. [Google Scholar]
- Stephenson, S.L. Changes in a former chesnut-dominated forest after a half century of succession. Am. Midl. Nat. 1986, 116, 173–179. [Google Scholar] [CrossRef]
- Storer, A.J.; Wood, D.L.; Gordon, T.R. Modification of coevolved insect-plant interactions by an exotic plant pathogen. Ecol. Entomol. 1999, 24, 238–243. [Google Scholar] [CrossRef]
- Brasier, C.M. Ophiostoma novo-ulmi sp. nove., causative agent of current Dutch elm disease pandemics. Mycopathologia, 1991, 115, 151–161. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Hulcr, J.; Wingfield, M.J.; de Beer, Z.W. Destructive tree diseases that are associated with ambrosia and bark beetles: Black swan events in tree pathology? Plant Dis. 2013, 95, 856–872. [Google Scholar] [CrossRef]
- Saunders, J.L.; Knoke, J.K. Diets for rearing the ambrosia beetle Xyleborus ferrugineus (Fabricius) in vitro. Science 1967, 157, 460. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Kajimura, H. Reproduction of the ambrosia beetle, Xyleborus pfeili (Ratzeburg) (Col., Scolytidae), on semi-artificial diet. J. Appl. Entomol. 2002, 126, 455–462. [Google Scholar] [CrossRef]
- Castrillo, L.A.; Griggs, M.H.; Vandenberg, J.D. Brood production by Xylosandrus germanus (Coleoptera: Curculionidae) and growth of its fungal symbiont on artificial diet based on sawdust of different tree species. Environ. Entomol. 2012, 41, 822–827. [Google Scholar] [CrossRef]
- Maner, M.L.; Hanula, J.L.; Braman, K. Rearing redbay ambrosia beetle, Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae), on semi-artificial media. Fla. Entomol. 2013, 96, 1042–1051. [Google Scholar] [CrossRef]
- Cooperband, M.F.; Stouthamer, R.; Carrillo, D.; Eskalen, A.; Thibault, T.; Cossé, A.A.; Castrillo, L.A.; Vanderberg, J.D.; Rugman-Jones, P.F. Biology of two members of the Euwallacea fornicatus species complex (Coleoptera: Curculionidae: Scolytinae), recently invasive in the U.S.A, reared on an ambrosia beetle artificial diet. Agric. For. Entomol. 2016, 18, 223–237. [Google Scholar] [CrossRef]
- Menocal, O.; Cruz, L.F.; Kendra, P.E.; Crane, J.H.; Ploetz, R.C.; Carrillo, D. Rearing Xyleborus volvulus (Coleoptera: Curculionidae) on media containing sawdust from avocado or silkbay, with or without Raffaelea lauricola (Ophiostomatales: Ophiostomataceae). Environ. Entomol. 2017, 46, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Castrillo, L.A.; Griggs, M.H.; Ranger, C.M.; Reding, M.E.; Vandenberg, J.D. Virulence of commercial strains of Beauveria bassiana and Metarhizium brunneum (Ascomycota: Hypocreales) against adult Xylosandrus germanus (Coleoptera: Curculionidae) and impact on brood. Biol. Control 2011, 58, 121–126. [Google Scholar] [CrossRef]
- Biedermann, P.H.W.; Klepzig, K.D.; Taborsky, M. Fungus cultivation by ambrosia beetles: Behavior and laboratory breeding success in three Xyleborine species. Environ. Entomol. 2009, 38, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, T.J.; Davis, J.M.; Harmon, C.L.; Ploetz, R.C.; Palmateer, A.J.; Soltis, P.S.; Smith, J.A. Development of multilocus PCR assays for Raffaelea lauricola, causal agent of laurel wilt disease. Plant Dis. 2014, 98, 379–383. [Google Scholar] [CrossRef]
- Harrington, T.C.; Aghayeva, D.N.; Fraedrich, S.W. New combinations in Raffaelea, Ambrosiella, and Hyalorhinocladiella, and four new species from the redbay ambrosia beetle, Xyleborus glabratus. Mycotaxon 2010, 111, 337–361. [Google Scholar] [CrossRef]
- Paine, T.D.; Raffa, K.F.; Harrington, T.C. Interactions among scolytid bark beetles, their associated fungi, and live host conifers. Ann. Rev. Entomol. 1997, 42, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Farrell, B.D.; Sequeira, A.S.; O’Meara, B.C.; Normark, B.B.; Chung, J.H.; Jordal, B.H. The evolution of agriculture in beetles (Curculionidae: Scolytinae and Platypodinae). Evolution 2001, 55, 2011–2027. [Google Scholar] [CrossRef] [PubMed]
- Mueller, U.G.; Gerardo, N.M.; Aanen, D.K.; Six, D.L.; Schultz, T.R. The evolution of agriculture in insects. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 563–595. [Google Scholar] [CrossRef]
- Beaver, R.A. Insect-fungus relationships in the bark and ambrosia beetles. In Insect-Fungus Interaction; Wilding, N., Collins, N.M., Hammond, P.M., Webber, J.F., Eds.; Academic Press: London, UK, 1989; pp. 121–143. [Google Scholar]
- Robinson, S.C.; Tudor, D.; Cooper, P.A. Wood preference of spalting fungi in urban hardwood species. Int. Biodeterior. Biodegr. 2011, 65, 1145–1149. [Google Scholar] [CrossRef]
- Abraham, L.D.; Roth, A.; Saddler, J.N.; Breuil, C. Growth, nutrition, and proteolytic activity of the sap-staining fungus Ophiostoma piceae. Can. J. Bot. 1993, 71, 1224–1230. [Google Scholar] [CrossRef]
- Käärik, A. Growth and sporulation of Ophiostoma and some other blueing fungi on synthetic media. Symb. Bot. Upsal. 1960, 16, 168. [Google Scholar]
- Merrill, W.; Cowling, E.B. Role of nitrogen in wood deterioration: Amount and distribution of nitrogen in fungi. Phytopathology 1966, 56, 1083–1090. [Google Scholar]
- French, J.R.; Roeper, R.A. Patterns of nitrogen utilization between the ambrosia beetle Xyleborus dispar and its symbiotic fungus. J. Insect Physiol. 1972, 78, 241–247. [Google Scholar] [CrossRef]
- Mizuno, T.; Kajimura, H. Effects of ingredients and structure of semi-artificial diet on the reproduction of an ambrosia beetle, Xyleborus pfeili (Ratzeburg) (Coleoptera: Curculionidae: Scolytinae). Appl. Entomol. Zool. 2009, 44, 363–370. [Google Scholar] [CrossRef]
- Saucedo, J.R.; Ploetz, R.C.; Konkol, J.L.; Ángel, M.; Mantilla, J.; Menocal, O.; Carrillo, D. Nutritional symbionts of a putative vector, Xyleborus bispinatus, of the laurel wilt pathogen of avocado, Raffaelea lauricola. Symbiosis 2017. [Google Scholar] [CrossRef]
- Bracewell, R.R.; Six, D.L. Experimental evidence of bark beetle adaptation to a fungal symbiont. Ecol. Evol. 2015, 5, 5109–5119. [Google Scholar] [CrossRef] [PubMed]
- Bleiker, K.P.; Potter, S.E.; Lauzon, C.R.; Six, D.L. Transport of fungal symbionts by mountain pine beetles. Can. Entomol. 2009, 141, 503–514. [Google Scholar] [CrossRef]
- Brar, G.S.; Capinera, J.L.; Kendra, P.E.; McLean, S.; Peña, J.E. Life cycle, development, and culture of Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae). Fla. Entomol. 2013, 96, 1158–1167. [Google Scholar] [CrossRef]
- Peer, K.; Taborsky, M. Outbreeding depression, but no inbreeding depression in haplodiploid ambrosia beetles with regular sibling mating. Evolution 2005, 59, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Entwistle, P.F. Inbreeding and arrhenotoky in the ambrosia beetle Xyleborus compactus (Eichh.) (Coleoptera: Scolytidae). Proc. R. Entomol. Soc. Lond. 1964, 39, 83–88. [Google Scholar] [CrossRef]
- Peer, K.; Taborsky, M. Female ambrosia beetles adjust their offspring sex ratio according to outbreeding opportunities for their sons. J. Evol. Biol. 2004, 17, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, P.H. Observations on sex ratio and behavior of males in Xyleborinus saxesenii Ratzeburg (Scolytinae, Coleoptera). ZooKeys 2010, 56, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, W.D. Extraordinary sex ratios. Science 1967, 156, 477–488. [Google Scholar] [CrossRef] [PubMed]



| Ingredients | Media | Manufacturer/Source | ||
|---|---|---|---|---|
| Type 1 AM1 or SM1 | Type 2 AM2 or SM2 | Type 3 AM3 or SM3 | ||
| Sawdust | 45 g | 84 g | 84 g | Avocado or silkbay wood |
| Granulated agar | 12 g | 12.6 g | 12.6 g | Difco Agar, Dickinson & Company, Sparks, MD, USA |
| Sucrose | 6 g | 2.1 g | 2.1 g | Fisher Scientific, Fair Lawn, NJ, USA |
| Starch | 3 g | 2.1 g | 2.1 g | Fisher Science Education, Nazareth, PA, USA |
| Yeast | 3 g | 2.1 g | 2.1 g | Fisher Science Education, Nazareth, PA, USA |
| Casein | 3 g | 4.2 g | 4.2 g | MP Biomedicals, LLC, Solon, OH, USA |
| Wesson’s salt mixture | 0.6 g | 0.52 g | 0.52 g | MP Biomedicals, LLC, Solon, OH, USA |
| Tetracycline | 0.21 g | 0.14 g | 0.14 g | Fisher Scientific, Fair Lawn, NJ, USA |
| Wheat germ oil | 1.5 mL | 1.05 mL | 1.05 mL | Frontier Scientific Services, Newark, DE, USA |
| Peanut oil | - | 1.05 mL | 1.05 mL | Ventura Foods, LLC, Brea, CA, USA |
| 95% ethanol | 3 mL | 2.1 mL | 2.1 mL | Decon Labs, Inc., King of Prussia, PA, USA |
| Distilled H2O | 370 mL | 244 mL | 540 mL | |
| Medium | Mean ± SE of Offspring per Tube after 40 Days | % of Females per Colony | N with Offspring (Any Stage) (%) | N with Females 1 (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Eggs | Larvae | Pupae | Male Adults | Female Adults | Brood (All Stages Combined) | ||||
| AM1 | 0.00 ± 0.00 | 4.08 ± 0.82 | 0.71 ± 0.26 | 0.96 ± 0.11 | 16.67 ± 1.27 | 22.42 ± 1.72 | 74% | 24 (100%) | 24 (100%) |
| AM1 + RL | 0.38 ± 0.24 | 5.17 ± 0.96 | 2.63 ± 0.69 | 1.58 ± 0.31 | 16.00 ± 2.00 | 25.75 ± 1.65 | 62% | 24 (100%) | 19 (79%) |
| SM1 | 0.17 ± 0.17 | 1.21 ± 0.32 | 0.25 ± 0.09 | 0.67 ± 0.14 | 10.13 ± 1.29 | 12.42 ± 1.39 | 82% | 24 (100%) | 24 (100%) |
| SM1 + RL | 0.21 ± 0.15 | 2.42 ± 0.60 | 0.58 ± 0.19 | 1.08 ± 0.18 | 9.54 ± 0.86 | 13.83 ± 0.53 | 69% | 24 (100%) | 21 (88%) |
| Medium | Mean ± SE of Offspring per Tube after 40 Days | % of Females per Colony | N with Offspring (Any Stage) (%) | N with Females 1 (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Eggs | Larvae | Pupae | Male Adults | Female Adults | Brood (All Stages Combined) | ||||
| AM2 | 1.08 ± 0.36 | 6.00 ± 0.99 | 1.63 ± 0.31 | 1.00 ± 0.17 | 16.00 ± 1.42 | 25.71 ± 1.94 | 62% | 23 (96%) | 23 (96%) |
| AM2 + RL | 1.50 ± 0.59 | 6.21 ± 0.66 | 1.58 ± 0.24 | 0.96 ± 0.11 | 9.75 ± 1.03 | 20.00 ± 1.65 | 49% | 24 (100%) | 21 (88%) |
| SM2 | 0.29 ± 0.22 | 4.79 ± 0.88 | 1.13 ± 0.26 | 0.83 ± 0.13 | 11.92 ± 1.61 | 18.96 ± 2.56 | 63% | 23 (96%) | 22 (92%) |
| SM2 + RL | 2.58 ± 0.67 | 8.63 ± 1.21 | 3.21 ± 0.34 | 1.04 ± 0.11 | 17.33 ± 1.85 | 32.79 ± 2.62 | 53% | 23 (96%) | 22 (92%) |
| Medium | Mean ± SE of Offspring per Tube after 40 Days | % of Females per Colony | N with Offspring (Any Stage) (%) | N with Females 1 (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Eggs | Larvae | Pupae | Male Adults | Female Adults | Brood (All Stages Combined) | ||||
| AM3 | 0.08 ± 0.05 | 1.21 ± 0.27 | 0.00 ± 0.00 | 0.79 ± 0.08 | 10.46 ± 0.91 | 12.54 ± 1.03 | 83% | 22 (92%) | 22 (92%) |
| AM3 + RL | 0.00 ± 0.00 | 1.13 ± 0.26 | 0.17 ± 0.10 | 0.83 ± 0.15 | 9.50 ± 0.86 | 11.63 ± 0.89 | 82% | 24 (100%) | 23 (96%) |
| SM3 | 0.04 ± 0.04 | 0.58 ± 0.30 | 0.17 ± 0.11 | 0.71 ± 0.09 | 5.71 ± 0.74 | 7.21 ± 0.99 | 79% | 20 (83%) | 20 (83%) |
| SM3 + RL | 0.00 ± 0.00 | 0.42 ± 0.21 | 0.21 ± 0.10 | 0.67 ± 0.10 | 5.83 ± 0.63 | 7.13 ± 0.79 | 82% | 23 (96%) | 23 (96%) |
| Medium Type 1 | Host | Mean No. of CFUs ± SE per Head & Pronotum | Frequency (n/N) | Mean No. of CFUs ± SE per Body Lacking Head & Pronotum | Frequency (n/N) |
|---|---|---|---|---|---|
| Medium 1 | Avocado | 18.9 ± 8.9 | 7/24 | 5.3 ± 3.3 | 3/24 |
| Silkbay | 9.7 ± 3.0 | 6/24 | 84 | 1/24 | |
| Medium 2 | Avocado | 6.7 ± 2.5 | 6/24 | 20 | 1/24 |
| Silkbay | 28.9 ± 10.3 | 10/24 | 2 | 1/24 | |
| Medium 3 | Avocado | 5.4 ± 2.9 | 5/24 | 2 | 1/24 |
| Silkbay | 6 ± 1.0 | 5/24 | 2 | 1/24 |
| Treatments | Species | Medium Containing Avocado Sawdust | Medium Containing Silkbay Sawdust | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Head and Pronotum | Body a | Gallery | Head and Pronotum | Body a | Gallery | ||||||
| Avg. CFU/Beetle | Freq. n/N | Avg. CFU/Beetle | Freq. n/N | Freq. n/N | Avg. CFU/Beetle | Freq. n/N | Avg. CFU/Beetle | Freq. n/N | Freq. n/N | ||
| Media inoculated with R. lauricola | Raffaelea arxii | 125 | 10/12 | 0 | 0/12 | 0/12 | 70.2 | 7/12 | 0 | 0/12 | 0/12 |
| Raffaelea lauricola | 4.67 | 3/12 | 0 | 0/12 | 8/12 | 23.8 | 6/12 | 23 | 1/12 | 6/12 | |
| Raffaelea subalba | 474.5 | 11/12 | 0 | 0/12 | 0/12 | 229 | 11/12 | 117 | 9/12 | 10/12 | |
| Raffaelea subfusca | 0 | 0/12 | 0 | 0/12 | 9/12 | 0 | 0/12 | 0 | 0/12 | 0/12 | |
| Phaeoacremonium inflatipes | 0 | 0/12 | 0 | 0/12 | 4/12 | 0 | 0/12 | 0 | 0/12 | 2/12 | |
| Candida multigemmis | 9.1 | 9/12 | 0 | 0/12 | 0/12 | 80.1 | 9/12 | 0 | 0/12 | 0/12 | |
| Media non-inoculated with R. lauricola | Raffaelea arxii | 40.5 | 9/12 | 0 | 0/12 | 0/12 | 42 | 3/12 | 0 | 0/12 | 0/12 |
| Raffaelea lauricola | 0 | 0/12 | 0 | 0/12 | 0/12 | 0 | 0/12 | 0 | 0/12 | 0/12 | |
| Raffaelea subalba | 199.7 | 11/12 | 0 | 0/12 | 0/12 | 253.1 | 12/12 | 0 | 0/12 | 0/12 | |
| Raffaelea subfusca | 35.2 | 12/12 | 0 | 0/12 | 10/12 | 0 | 0/12 | 0 | 0/12 | 11/12 | |
| Phaeoacremonium inflatipes | 0 | 0/12 | 0 | 0/12 | 0/12 | 0 | 0/12 | 0 | 0/12 | 4/12 | |
| Candida multigemmis | 37.3 | 10/12 | 0 | 0/12 | 0/12 | 29.7 | 10/12 | 0 | 0/12 | 0/12 | |
| Alloascoidea sp. | 0 | 0/12 | 0 | 0/12 | 6/12 | 0 | 0/12 | 0 | 0/12 | 0/12 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menocal, O.; Cruz, L.F.; Kendra, P.E.; Crane, J.H.; Cooperband, M.F.; Ploetz, R.C.; Carrillo, D. Xyleborus bispinatus Reared on Artificial Media in the Presence or Absence of the Laurel Wilt Pathogen (Raffaelea lauricola). Insects 2018, 9, 30. https://doi.org/10.3390/insects9010030
Menocal O, Cruz LF, Kendra PE, Crane JH, Cooperband MF, Ploetz RC, Carrillo D. Xyleborus bispinatus Reared on Artificial Media in the Presence or Absence of the Laurel Wilt Pathogen (Raffaelea lauricola). Insects. 2018; 9(1):30. https://doi.org/10.3390/insects9010030
Chicago/Turabian StyleMenocal, Octavio, Luisa F. Cruz, Paul E. Kendra, Jonathan H. Crane, Miriam F. Cooperband, Randy C. Ploetz, and Daniel Carrillo. 2018. "Xyleborus bispinatus Reared on Artificial Media in the Presence or Absence of the Laurel Wilt Pathogen (Raffaelea lauricola)" Insects 9, no. 1: 30. https://doi.org/10.3390/insects9010030
APA StyleMenocal, O., Cruz, L. F., Kendra, P. E., Crane, J. H., Cooperband, M. F., Ploetz, R. C., & Carrillo, D. (2018). Xyleborus bispinatus Reared on Artificial Media in the Presence or Absence of the Laurel Wilt Pathogen (Raffaelea lauricola). Insects, 9(1), 30. https://doi.org/10.3390/insects9010030