Within-Host Competition between Two Entomopathogenic Fungi and a Granulovirus in Diatraea saccharalis (Lepidoptera: Crambidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect Colony
2.2. Entomopathogens
2.3. Virulence Bioassays for Interspecific within-Host Competition
2.4. Statistical Analysis
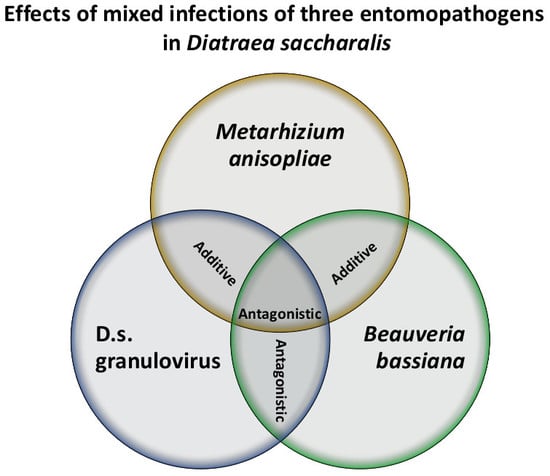
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Read, A.F.; Taylor, L.H. The ecology of genetically diverse infections. Science 2001, 292, 99–1102. [Google Scholar] [CrossRef]
- Cox, F.E.G. Concomitant infections, parasites and immune responses. Parasitology 2001, 122, S23–S38. [Google Scholar] [CrossRef] [PubMed]
- Goertz, D.; Hoch, G. Three microsporidian pathogens infecting Lymantria dispar larvae do not differ in their success in horizontal transmission. J. Appl. Entomol. 2009, 133, 568–570. [Google Scholar] [CrossRef]
- Ma, X.; Liu, X.; Ning, X.; Zhang, B.; Han, F.; Guan, X.; Tan, Y.; Zhang, Q. Effects of Bacillus thuringiensis toxin Cry1Ac and Beauveria bassiana on Asiatic corn borer (Lepidoptera: Crambidae). J. Invertebr. Pathol. 2008, 99, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Marzban, R.; He, Q.; Liu, X.; Zhang, Q. Effects of Bacillus thuringiensis toxin Cry1Ac and cytoplasmic polyhedrosis virus of Helicoverpa armigera (Hübner) (HaCPV) on cotton bollworm (Lepidoptera: Noctuidae). J. Invertebr. Pathol. 2009, 101, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Raymond, B.; Lijek, R.S.; Griffiths, R.I.; Bonsall, M.B. Ecological consequences of ingestion of Bacillus cereus on Bacillus thuringiensis infections and on the gut flora of a lepidopteran host. J. Invertebr. Pathol. 2008, 99, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Tounou, A.K.; Kooyman, C.; Douro-Kpindou, O.D.; Poehling, H.M. Combined field efficacy of Paranosema locustae and Metarhizium anisopliae var. acridum for the control of Sahelian grasshoppers. Biocontrol 2008, 53, 813–828. [Google Scholar] [CrossRef]
- Hughes, W.O.H.; Boomsma, J.J. Let your enemy do the work: Within-host interactions between two fungal parasites of leaf-cutting ants. Biol. Lett. 2004, 271, S104–S106. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Franco, A.W.; Atkins, S.D.; Clark, S.J.; Alderson, P.J.; Pell, J.K. Use of quantitative PCR to understand within-host competition between two entomopathogenic fungi. J. Invertebr. Pathol. 2011, 107, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Inglis, G.D.; Johnson, D.L.; Cheng, K.J.; Goettel, M.S. Use of pathogen combinations to overcome the constraints of temperature on entomopathogenic hyphomycetes against grasshoppers. Biol. Control 1997, 8, 143–152. [Google Scholar] [CrossRef]
- Inglis, G.D.; Duke, G.M.; Kawchuk, L.M.; Goettel, M.S. Influence of oscillating temperatures on the competitive infection and colonization of the migratory grasshopper by Beauveria bassiana and Metarhizium flovoviride. Biol. Control 1999, 14, 111–120. [Google Scholar] [CrossRef]
- Rao, C.U.M.; Uma Devi, H.; Khan, P.A.A. Effect of combination treatment with entomopathogenic fungi Beauveria bassiana and Nomuraea rileyi (Hypocerales) on Spodoptera litura (Lepidoptera: Noctuidae). Biocontrol Sci. Technol. 2006, 16, 221–232. [Google Scholar]
- Thomas, M.B.; Watson, E.L.; Valverde-Garcia, P. Mixed infections and insect-pathogen interactions. Ecol. Lett. 2003, 6, 183–188. [Google Scholar] [CrossRef]
- União dos Produtores de Bioenergia (UPON). Evolução da Produtividade da Cana-de Açúcar: Safras 2005/2006 a 2010/2011. 2017. Available online: http://www.udop.com.br/download/estatistica/area_cultivada/13mar18_area_colhida_evolucao_produtividade.pdf (accessed on 4 April 2018).
- Dinardo-Miranda, L.L. Pragas. In Cana-de-Açúcar; Dinardo-Miranda, L.L., Vasconceles, A.C.M., Landell, M.G.A., Eds.; Instituto Agronômico: Campinas, Brazil, 2008. [Google Scholar]
- Boomsma, J.J.; Jensen, A.B.; Meyling, N.V.; Jørgen Eilenberg, J. Evolutionary Interaction Networks of Insect Pathogenic Fungi. Annu. Rev. Entomol. 2014, 59, 467–485. [Google Scholar] [CrossRef] [PubMed]
- Humber, R.A. Evolution of entomopathogenicity in Fungi. J. Invertebr. Pathol. 2008, 98, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Mascarin, G.M.; Lopes, R.B.; Delalibera, I., Jr.; Fernandes, E.K.K.; Luz, C.; Faria, M. Current status and perspectives of fungal entomopathogens used for microbial control of arthropod pests in Brazil. J. Invertebr. Pathol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ardisson-Araújo, D.M.P.; Pereira, B.T.; Melo, F.L.; Ribeiro, B.M.; Báo, S.N.; Zanotto, P.M.A.; Moscardi, F.; Kitajima, E.W.; Sosa-Gomez, D.R.; Wolff, J.L.C. A betabaculovirus encoding a gp64 homolog. BMC Genom. 2016, 7, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, W.O.H.; Petersen, K.S.; Ugelvig, L.V.; Pedersen, D.; Thomsen, L.; Poulsen, M.; Boomsma, J.J. Density-dependence and within-host competition in a semelparous parasite of leaf-cutting ants. Evol. Biol. 2004, 4, 45–57. [Google Scholar]
- Alves, S.B. Controle Microbiano de Insetos; FEALQ: Piracicaba, Brazil, 1998; p. 407. ISBN 85-7133-004-2. [Google Scholar]
- Lecuona, R.E.; Alves, S.B. Efficiency of Beauveria bassiana (Bals.) Vuill., B. brongniartii (Sacc.) Petch. and granulose virus on Diatraea saccharalis (F.; 1794) at different temperatures. J. Appl. Entomol. 1998, 105, 223–228. [Google Scholar] [CrossRef]
- Hensley, S.D.; Hammond, A.H. Laboratory techniques for rearing the sugarcane borer on an artificial diet. J. Econ. Entomol. 1968, 61, 1742–1743. [Google Scholar] [CrossRef]
- Collett, D. Modeling Binary Data; Chapman & Hall: New York, NY, USA, 1991; p. 369. ISBN 9781584883241. [Google Scholar]
- SAS Institute. SAS/STAT® User Guide, version 9.1; SAS Institute: Cary, NC, USA, 2003. [Google Scholar]
- Robertson, J.L.; Preisler, H.K. Pesticide Bioassays with Arthropods; CRC Press: Boca Raton, FL, USA, 1992; p. 127. [Google Scholar]
- Tabashnik, B.E. Evaluation of synergism among Bacillus thuringiensis toxins. Appl. Environ. Microbiol. 1992, 58, 3343–3346. [Google Scholar] [PubMed]
- SPSS. SPSS Statistics for Windows, version 17.0; SPSS Inc.: Chicago, IL, USA, 2008. [Google Scholar]
- Leal-Bertioli, S.C.M.; Butt, T.M.; Peberdy, J.F.; Bertioli, D.J. Genetic exchange in Metarhizium anisopliae strains co-infecting Phaedon cochleariae is revealed by molecular markers. Mycol. Res. 2000, 104, 409–414. [Google Scholar] [CrossRef]
- Wang, C.S.; Li, Z.Z.; Butt, T.M. Molecular studies of co-formulated strains of the entomopathogenic fungus, Beauveria bassiana. J. Invertebr. Pathol. 2002, 80, 29–34. [Google Scholar] [CrossRef]
- Koppenhöfer, A.M.; Kaya, H.K. Additive and synergist interaction between entomopathogenic nematodes and Bacillus thuringiensis for scarab grub control. Biol. Control 1997, 8, 131–137. [Google Scholar] [CrossRef]
- Malakar, R.; Elkinton, J.S.; Hajek, A.E.; Burand, J.P. Within-host interactions of Lymantria dispar (Lepidoptera: Lymantriidae) nucleopolyhedrosis virus and Entomophaga maimaiga (Zygomycets: Entomophthorales). J. Invertebr. Pathol. 1999, 73, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Wraight, S.P.; Ramos, M.E. Synergistic interaction between Beauveria bassiana and Bacillus thuringiensis tenebrionis based biopesticides applied against field populations of Colorado potato beetle larvae. J. Invertebr. Pathol. 2005, 90, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Franco, A.W.; Clark, S.J.; Alderson, P.G.; Pell, J.K. Competition and co-existence of Zoophthora radicans and Pandora blunckii, two co-occurring fungal pathogens of the diamondback moth, Plutella xylostella. Mycol. Res. 2009, 113, 1312–1321. [Google Scholar] [CrossRef] [PubMed]

| Inoculum | N | df | a χ2 | Slope ± SE | Intercept ± SE | b h | LC50 Observed (95% CI) | c LC50 Expected |
|---|---|---|---|---|---|---|---|---|
| Ma | 300 | 28 | 32.41 | 1.54 ± 0.24 ** | −11.9 ± 1.9 | 1.2 | 5.61 × 107 (3.1 × 107; 9.1 × 107) | - |
| Bb | 300 | 28 | 39.75 | 1.02 ± 0.24 ** | −7.3 ± 1.9 | 1.42 | 1.35 × 107 (1.86 × 106; 3.3 × 107) | - |
| DisaGV | 300 | 28 | 23.61 | 1.12 ± 0.20 ** | −8.4 ± 1.6 | 1.0 | 3.36 × 107 (1.4 × 107; 6.0 × 107) | - |
| Ma + Bb | 300 | 28 | 30.12 | 1.04 ± 0.21 ** | −7.5 ± 1.7 | 1.08 | 1.40 × 107 (3.1 × 106; 3.1 × 107) | 2.17 × 107 Additive |
| Ma + DisaGV | 300 | 28 | 29.10 | 0.95 ± 0.19 ** | −7.0 ± 1.6 | 1.04 | 2.7 × 107 (7.7 × 106; 5.6 × 107) | 4.21 × 107 Additive |
| Bb + DisaGV | 300 | 28 | 26.30 | 1.32 ± 0.21 ** | −10.7 ± 1.7 | 1.0 | 1.22 × 108 (7.4 × 107; 2.0 × 108) | 1.92 × 107 Antagonistic |
| Ma + Bb + DisaGV | 300 | 28 | 34.02 | 1.41 ± 0.24 ** | −11.2 ± 1.9 | 1.22 | 8.5 × 107 (4.7 × 107; 1.5 × 108) | 2.49 × 107 Antagonistic |
| Treatment | ST50 (95% FL) b | χ2 | p-Value | |
|---|---|---|---|---|
| Pathogen a | Concentration | |||
| Ma | 107 | --- | --- | --- |
| 5 × 107 | --- | --- | --- | |
| 1 × 108 | 6.5 (5.4; 7.7) | 141.10 | <0.0001 | |
| 5 × 108 | 6.2 (5.6; 6.8) | 73.31 | 0.2787 | |
| 1 × 109 | 3.7 (3.2; 4.2) | 102.96 | 0.0031 | |
| Bb | 107 | --- | --- | --- |
| 5 × 107 | 9.9 (9.1; 10.8) | 123.68 | <0.0001 | |
| 1 × 108 | 7.6 (6.8; 8.4) | 120.01 | <0.0001 | |
| 5 × 108 | 7.0 (6.2; 7.9) | 190.28 | <0.0001 | |
| 1 × 109 | 5.3 (4.7; 6.0) | 152.76 | <0.0001 | |
| Ma + Bb | 107 | 9.6 (8.9; 10.5) | 91.33 | 0.0258 |
| 5 × 107 | 9.0 (8.1; 10.2) | 134.76 | <0.0001 | |
| 1 × 108 | 7.1 (6.3; 8.1) | 101.97 | 0.0038 | |
| 5 × 108 | 6.9 (6.3; 7.6) | 80.18 | 0.1296 | |
| 1 × 109 | 5.7 (5.1; 6.2) | 169.61 | <0.0001 | |
| d DisaGV | 107 | --- | --- | --- |
| 5 × 107 | --- | --- | --- | |
| 1 × 108 | 10.0 (9.3; 10.7) | 98.47 | 0.0074 | |
| 5 × 108 | 11.0 (10.4; 11.5) | 43.45 | 0.9886 | |
| 1 × 109 | 8.8 (8.4; 9.3) | 64.99 | 0.5468 | |
| Ma + DisaGV | 107 | --- | --- | --- |
| 5 × 107 | 11.3 (10.5; 12.3) | 68.48 | 0.4267 | |
| 1 × 108 | 11.6 (10.9; 12.6) | 76.93 | 0.1904 | |
| 5 × 108 | 9.2 (8.5; 10.0) | 63.58 | 0.5957 | |
| 1 × 109 | 7.4 (6.7; 8.2) | 217.60 | <0.0001 | |
| Bb + DisaGV | 107 | --- | --- | --- |
| 5 × 107 | --- | --- | --- | |
| 1 × 108 | 10.9 (10.2; 11.7) | 110.97 | 0.0006 | |
| 5 × 108 | 10.6 (9.9; 11.3) | 94.46 | 0.0152 | |
| 1 × 109 | 9.0 (8.3; 9.9) | 86.70 | 0.0531 | |
| Ma + Bb + DisaGV | 107 | --- | --- | --- |
| 5 × 107 | --- | --- | --- | |
| 1 × 108 | 10.2 (9.4; 11.3) | 85.88 | 0.0599 | |
| 5 × 108 | 7.4 (6.5; 8.5) | 150.36 | <0.0001 | |
| 1 × 109 | 7.4 (6.8; 7.9) | 115.41 | 0.0002 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauli, G.; Moura Mascarin, G.; Eilenberg, J.; Delalibera Júnior, I. Within-Host Competition between Two Entomopathogenic Fungi and a Granulovirus in Diatraea saccharalis (Lepidoptera: Crambidae). Insects 2018, 9, 64. https://doi.org/10.3390/insects9020064
Pauli G, Moura Mascarin G, Eilenberg J, Delalibera Júnior I. Within-Host Competition between Two Entomopathogenic Fungi and a Granulovirus in Diatraea saccharalis (Lepidoptera: Crambidae). Insects. 2018; 9(2):64. https://doi.org/10.3390/insects9020064
Chicago/Turabian StylePauli, Giuliano, Gabriel Moura Mascarin, Jørgen Eilenberg, and Italo Delalibera Júnior. 2018. "Within-Host Competition between Two Entomopathogenic Fungi and a Granulovirus in Diatraea saccharalis (Lepidoptera: Crambidae)" Insects 9, no. 2: 64. https://doi.org/10.3390/insects9020064
APA StylePauli, G., Moura Mascarin, G., Eilenberg, J., & Delalibera Júnior, I. (2018). Within-Host Competition between Two Entomopathogenic Fungi and a Granulovirus in Diatraea saccharalis (Lepidoptera: Crambidae). Insects, 9(2), 64. https://doi.org/10.3390/insects9020064