New Twin-Roll Cast Al-Li Based Alloys for High-Strength Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
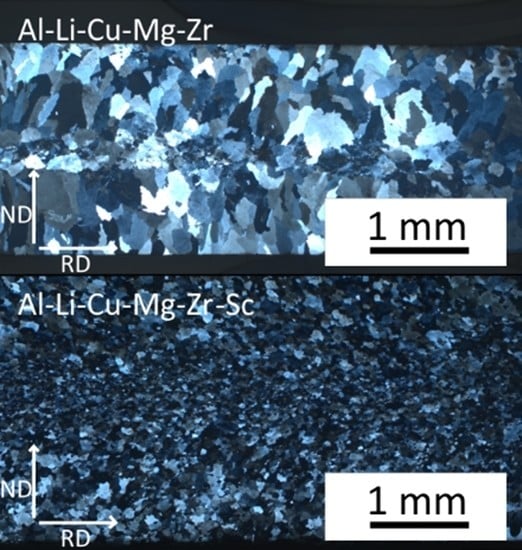
3.1. Microstructure of As-Cast Materials
3.2. Evolution of Microstructure during Heating
4. Conclusions
- Strips of two Al-Li based alloys with and without the addition of Sc were produced by twin-roll casting.
- A fine dendritic structure forms during the processing by twin-roll casting.
- Sc alloying results in the formation of finer grains already during casting.
- Al3Sc1-xZrx particles precipitate in the Sc-containing alloy at temperatures near 400 °C.
- The as-cast grain structure is replaced by a recrystallized and coarsened structure in the Sc-free alloy at temperatures close to 500 °C.
- The presence of Al3Sc1-xZrx particles effectively inhibits recrystallization, and even at the highest annealing temperatures no grain growth occurs in the Al-Li-Cu-Mg-Zr-Sc alloy.
- Primary phases formed during casting could not dissolve below 500 °C, which could limit the properties of new twin-roll cast alloys.
Author Contributions
Funding
Conflicts of Interest
References
- Abd El-Aty, A.; Xu, Y.; Zhang, S.H.; Ma, Y.; Chen, D.Y. Experimental investigation of tensile properties and anisotropy of 1420, 8090 and 2060 Al-Li alloys sheet undergoing different strain rates and fibre orientation: A comparative study. Procedia Eng. 2017, 207, 13–18. [Google Scholar] [CrossRef]
- Barekar, N.S.; Das, S.; Yang, X.; Huang, Y.; El Fakir, O.; Bhagurkar, A.G.; Zhou, L.; Fan, Z. The impact of melt conditioning on microstructure, texture and ductility of twin roll cast aluminium alloy strips. Mater. Sci. Eng. A 2016, 650, 365–373. [Google Scholar] [CrossRef]
- Rioja, R.; Liu, J. The evolution of Al-Li base products for aerospace and space applications. Metall. Mater. Trans. A 2012, 43, 3325–3337. [Google Scholar] [CrossRef]
- Lavernia, E.J.; Srivatsan, T.S.; Mohamed, F.A. Strength, deformation, fracture behaviour and ductility of aluminium-lithium alloys. J. Mater. Sci. 1990, 25, 1137–1158. [Google Scholar] [CrossRef]
- Prasad, N.E.; Gokhale, A.A.; Rao, P.R. Mechanical behaviour of aluminium-lithium alloys. Sadhana 2003, 28, 209–246. [Google Scholar] [CrossRef]
- Alexopoulos, N.; Migklis, E.; Stylianos, A.; Myriounis, D.P. Fatigue behavior of the aeronautical Al–Li (2198) aluminum alloy under constant amplitude loading. Int. J. Fatigue 2013, 56, 95–105. [Google Scholar] [CrossRef]
- Kashyap, B.; Chaturvedi, M. Stain anisotropy in AA8090 Al–Li alloy during high temperature deformation. Mater. Sci. Eng. A 2020, 281, 88–95. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, H.; Wu, H. The evolution of precipitation and microstructure in friction stir welded 2195-T8 Al-Li alloy. Mater. Sci. Eng. A 2015, 625, 322–329. [Google Scholar] [CrossRef]
- Lavernia, E.J.; Grant, N.J. Aluminium-lithium alloys. J. Mater. Sci. 1987, 22, 1521–1529. [Google Scholar] [CrossRef]
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Prasad, K.; Prasad, N.; Gokhale, A. Microstructure and precipitate characteristics of aluminum-lithium alloys. In Aluminum-Lithium Alloys: Processing, Properties, and Applications; Prasad, N.E., Gokhale, A., Wanhill, H., Eds.; Elsevier Inc.: Oxford, UK, 2014; pp. 99–137. [Google Scholar]
- Srivatsan, T.; Lavernia, E.; Eswara Prasad, N.; Kutumbarao, V. Quasi-static strength, deformation, and fracture behavior of aluminum-lithium alloys. In Aluminum-Lithium Alloys: Processing, Properties, and Applications; Prasad, N.E., Gokhale, A., Wanhill, H., Eds.; Elsevier Inc.: Oxford, UK, 2014; pp. 305–339. [Google Scholar]
- Ashton, R.F.; Thompson, D.S.; Gayle, F.W. The effect of processing on the properties of Al-Li alloys. In Aluminum Alloys-Their Physical and Mechanical, Properties; Starke, J., Sanders, J., Eds.; EMAS: Warley, UK, 1986; pp. 403–417. [Google Scholar]
- Noble, B.; Harris, S.J.; Dinsdale, K. Yield characteristics of aluminium–lithium alloys. Met. Sci. 1982, 16, 425–430. [Google Scholar] [CrossRef]
- Lin, F.; Chakraborty, S.; Starke, E. Microstructure-property relationships of two Al-3Li-2Cu-0.2Zr-X Cd alloys. Metall. Trans. A 1982, 13, 401–410. [Google Scholar] [CrossRef]
- Suresh, S.; Vasudevan, A.; Tosten, M.; Howell, P. Microscopic and macroscopic aspects of fracture in lithium containing aluminium alloys. Acta Metall. 1987, 35, 25–46. [Google Scholar] [CrossRef]
- Srivatsan, T.S.; Place, T.A. Microstructure, tensile properties and fracture behaviour of an Al-Cu-Li-Mg-Zr alloy 8090. J. Mater. Sci. 1989, 24, 1543–1551. [Google Scholar] [CrossRef]
- Jata, K.V.; Starke, E.A. Fatigue crack growth and fracture toughness behavior of an Al-Li-Cu alloy. Metall. Trans. A 1986, 17, 1011–1026. [Google Scholar] [CrossRef]
- Peel, C.; McDarmaid, D.; Evans, B. Considerations of critical factors for the design of aerospace structures using current and future Al-Li alloys. In Aluminium_Lithium Alloys: Design, Development and Applications Update; Kar, S.J., Agarwal, R., Quist, S., Eds.; ASM: International Park, OH, USA, 1988; pp. 315–337. [Google Scholar]
- Marquis, E.A.; Seidman, D.N. Nanoscale structural evolution of Al3Sc precipitates in Al(Sc) alloys. Acta Mater. 2001, 49, 1909–1919. [Google Scholar] [CrossRef] [Green Version]
- Ferry, M. Direct Strip Casting of Metals and Alloys, 1st ed.; Woodhead Publishing Limited: Cambridge, UK, 2006. [Google Scholar]
- Zhu, Y.T.; Jiang, H.; Huang, J.; Lowe, T.C. A new route to bulk nanostructured metals. Metall. Mater. Trans. A 2001, 32, 1559–1562. [Google Scholar] [CrossRef]
- Shin, D.H.; Park, J.J.; Kim, Y.S.; Park, K.T. Constrained groove pressing and its application to grain refinement of aluminum. Mater. Sci. Eng. A 2002, 328, 98–103. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Estrin, Y.; Horita, Z.; Langdon, T.G.; Zehetbauer, M.J.; Zhu, Y.T. Producing bulk ultrafine-grained materials by severe plastic deformation. JOM 2006, 58, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Barekar, N.S.; Dhindaw, B.K. Twin-roll casting of aluminum alloys—An overview. Mater. Manuf. Process. 2014, 29, 651–661. [Google Scholar] [CrossRef]
- Wang, L.; Yu, H.; Lee, Y.S.; Kim, M.S.; Kim, H.W. Effect of microstructure on hot tensile deformation behavior of 7075 alloy sheet fabricated by twin roll casting. Mater. Sci. Eng. A 2016, 652, 221–230. [Google Scholar] [CrossRef]
- Park, Y.S.; Lee, S.B.; Kim, N.J. Microstructure and mechanical properties of strip cast Al-Mg-Si-X alloys. Mater. Trans. 2003, 44, 2617–2624. [Google Scholar] [CrossRef] [Green Version]
- Birol, Y. Analysis of macro segregation in twin-roll cast aluminium strips via solidification curves. J. Alloys Compd. 2009, 486, 168–172. [Google Scholar] [CrossRef]
- Birol, Y. Homogenization of a twin-roll cast thin Al–Mn strip. J. Alloys Compd. 2009, 471, 122–127. [Google Scholar] [CrossRef]
- Altuner, H.M.; Işıksaçan, C.; Birbaşar, O.; Günyüz, M.; Meydanoğlu, O. Crystallographic texture development of As-Cast 3105 alloy produced by St/Cu shell pair. In Light Metals; Williams, E., Ed.; Springer: Cham, Switzerland, 2016; pp. 1025–1030. [Google Scholar] [CrossRef]
- Schmidt, C.W.; Mortensen, D.; Karhausen, K.F. Influence of Process Conditions on Segregation Behavior in Twin-Roll Casting of an AlFeSi-Alloy. In Light Metals; Springer: Cham, Switzerland, 2017; pp. 811–819. [Google Scholar] [CrossRef]
- Hoseinifar, A.; Salari, S.; Nezhad, M. Effect of twin-roll casting parameters on microstructure and mechanical properties of AA5083-H321 sheet. Trans. Nonferrous Met. Soc. China 2016, 26, 2552–2560. [Google Scholar] [CrossRef]
- Haga, T.; Tkahashi, K.; Ikawaand, M.; Watari., H. Twin roll casting of aluminum alloy strips. J. Mater. Process. Technol. 2004, 153–154, 42–47. [Google Scholar] [CrossRef]
- Birol, Y. Pre-aging to improve bake hardening in a twin-roll cast Al–Mg–Si alloy. Mater. Sci. Eng. A 2005, 391, 175–180. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Li, J.D.; Gao, G.J.; Wang, Z.D. 6000 series aluminium alloy strip casting using a vertical type twin roll caster. Mater. Sci. Forum 2016, 877, 45–50. [Google Scholar] [CrossRef]
- Grydin, O.; Schaper, M.; Danchenko, V. Twin-roll casting of high-strength age-hardened aluminium alloys. Metall. Min. Ind. 2011, 3, 7–16. [Google Scholar]
- Cieslar, M.; Bajer, J.; Zimina, M.; Šlapáková, M.; Grydin, O. Properties and microstructure of twin-roll cast Al-Mg alloy containing Sc and Zr. In Proceedings of the 4th International Conference Recent Trends in Structural Materials, Pilsen, Czech Republic, 9–11 November 2016; IOP Publishing Ltd.: Bristol, UK, 2017. [Google Scholar]
- Li, S.; He, C.; Fu, J.; Xu, J.; Xu, G.; Wang, Z. Evolution of microstructure and properties of novel aluminum-lithium alloy with different roll casting process parameters during twin-roll casting. Mater. Charact. 2020, 161, 110145. [Google Scholar] [CrossRef]
- Grydin, O.; Ogins’kyy, Y.K.; Danchenko, V.M.; Bach, F.W. Experimental twin-roll casting equipment for production of thin strips. Metall. Min. Ind. 2010, 2, 348–354. [Google Scholar]
- Grydin, O.; Stolbchenko, M.; Nürnberger, F.; Schaper, M. Influence of the twin-roll casting parameters on the microsegregation in thin strips of the aluminium alloy EN AW-6082. In Light Metals 2014; Springer: Cham, Switzerland, 2014; pp. 411–414. [Google Scholar]
- Grydin, O.; Stolbchenko, M.; Schaper, M. Influence of nozzle shape on near-surface segregation formation during twin-roll casting of aluminum strips. In Light Metals 2020; Springer: Cham, Switzerland, 2020; pp. 1039–1044. [Google Scholar]
- Yun, M.; Lokyer, S.; Hunt, J.D. Twin roll casting of aluminium alloys. Mater. Sci. Eng. A 2000, 280, 116–123. [Google Scholar] [CrossRef]
- Song, R.; Harada, Y.; Murashi, S.; Kumai, S. Microstructural evolution of Al-Mn based alloy fabricated by vertical-type high-speed twin-roll casting. Mater. Sci. Forum 2016, 877, 51–55. [Google Scholar] [CrossRef]
- Lockyer, S.A.; Yun, M.; Hunt, J.D.; Edmonds, D.V. Micro- and macrodefects in thin sheet twin-roll cast aluminum alloys. Mater. Charact. 1996, 37, 301–310. [Google Scholar] [CrossRef]
- Monaghan, D.J.; Henderson, M.B.; Hunt, J.D.; Edmonds, D.V. Microstructural defects in high productivity twin-roll casting of aluminium. Mater. Sci. Eng. A 1993, 173, 251–254. [Google Scholar] [CrossRef]
- Rioja, R.J. Fabrication methods to manufacture isotropic Al-Li alloys and products for space and aerospace applications. Mater. Sci. Eng. A 1998, 257, 100–107. [Google Scholar] [CrossRef]
- Araullo-Peters, V.; Gault, B.; de Gauser, F.; Dechamps, A.; Cairney, J.M. Microstructural evolution during ageing of Al–Cu–Li–x alloys. Acta Mater. 2014, 66, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.P.; Zheng, Z.Q. Independent and combined roles of trace Mg and Ag additions in properties precipitation process and precipitation kinetics of Al−Cu−Li−(Mg)−(Ag) −Zr−Ti alloys. Acta Mater. 1998, 46, 4381–4393. [Google Scholar] [CrossRef]
- Hájek, M.; Veselý, J.; Cieslar, M. Precision of electrical resistivty measurements. Mater. Scie. Eng. A 2007, 462, 339–342. [Google Scholar] [CrossRef]
- Decreaux, B.; Deschamps, A.; de Geuser, F.; Donnadieu, P.; Sigli, C.; Weyland, M. The influence of Cu/Li ratio on precipitation in Al–Cu–Li–x alloys. Acta Mater. 2013, 61, 2207–2218. [Google Scholar] [CrossRef]
- Nes, E.; Ryum, N.; Hunderi, O. On the Zener drag. Acta Metall. 1985, 33, 11–22. [Google Scholar] [CrossRef]
- Romios, M.; Tiraschi, R.; Parrish, C.; Babel, H.W.; Ogren, J.R.; Es-Said, O.S. Design of multistep aging treatments of 2099(C458) Al–Li alloy. J. Mater. Eng. Perform 2005, 14, 641–646. [Google Scholar] [CrossRef]










| Element | Cu | Li | Mg | Zr | Sc | Ag | Ti | V | Fe | Al | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content, wt.% | 3.71 | 0.91 | 0.31 | 0.15 | 0 | 0.33 | 0.02 | 0.01 | 0.05 | 94.49 | Balance |
| Without Sc Addition | Cu | Li | Mg | Zr | Sc | Ag | Ti | V | Fe | Al | Other |
| Content, wt.% | 2.52 | 0.72 | 0.27 | 0.12 | - | 0.33 | 0.02 | 0.01 | 0.09 | 95.99 | Balance |
| With Sc addition | Cu | Li | Mg | Zr | Sc | Ag | Ti | V | Fe | Al | Other |
| Content, wt.% | 2.61 | 0.71 | 0.27 | 0.10 | 0.17 | 0.29 | 0.01 | 0.01 | 0.09 | 95.32 | Balance |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grydin, O.; Stolbchenko, M.; Schaper, M.; Belejová, S.; Králík, R.; Bajtošová, L.; Křivská, B.; Hájek, M.; Cieslar, M. New Twin-Roll Cast Al-Li Based Alloys for High-Strength Applications. Metals 2020, 10, 987. https://doi.org/10.3390/met10080987
Grydin O, Stolbchenko M, Schaper M, Belejová S, Králík R, Bajtošová L, Křivská B, Hájek M, Cieslar M. New Twin-Roll Cast Al-Li Based Alloys for High-Strength Applications. Metals. 2020; 10(8):987. https://doi.org/10.3390/met10080987
Chicago/Turabian StyleGrydin, Olexandr, Mykhailo Stolbchenko, Mirko Schaper, Sára Belejová, Rostislav Králík, Lucia Bajtošová, Barbora Křivská, Michal Hájek, and Miroslav Cieslar. 2020. "New Twin-Roll Cast Al-Li Based Alloys for High-Strength Applications" Metals 10, no. 8: 987. https://doi.org/10.3390/met10080987
APA StyleGrydin, O., Stolbchenko, M., Schaper, M., Belejová, S., Králík, R., Bajtošová, L., Křivská, B., Hájek, M., & Cieslar, M. (2020). New Twin-Roll Cast Al-Li Based Alloys for High-Strength Applications. Metals, 10(8), 987. https://doi.org/10.3390/met10080987