Strengthening of Nanocrystalline Al with Al3Zr Core-Shell Structure
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
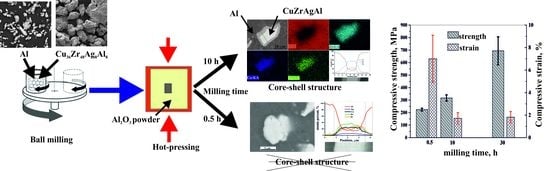
3.1. Morphology of the Milled Al-CuZrAgAl Composite Powder Particles
3.2. Microstructure of the Al-Based Composite Powder Particles
3.3. Thermal Analysis of the Al-Based Composite Powder Material
3.4. Microstructure of the Hot-Pressed Composites
3.5. Mechanical Properties of the Hot-Pressed Composites
4. Conclusions
- A core-shell structure has been developed by the surface interdiffusion between Al and Zr, during the hot-pressing independently of the PCA use. The shell consists of tetragonal Al3Zr phase based on TEM investigation. However, the formation of the core-shell structure requires long-term high-energy milling before the pressing. A prolonged milling process provides good surface activity for its formation.
- The increased mechanical properties are ascribed to the homogeneously distributed particles with core-shell structure, high relative density, the good interfacial bonding, and grain refinement strengthening. A very strong interfacial bonding exists between the reinforcement and the matrix.
- The core-shell structure is coarsening resistant because the shell is connected to the core.
- The shell and the crystallite size being reduced significantly improves the compressive strength of the composites up to 694 ± 112 MPa at room temperature. Even at 400 °C, the compressive strength is 380 MPa, which is very significant at this temperature.
- The Brinell hardness of bulk pressed samples after 10 and 30 h milling are in the range of 275 and 329 HB, respectively.
- The PCA has a great effect on the milling process; however, the effect of the core-shell structure is more pronounced in the case of the sintered samples.
Funding
Acknowledgments
Conflicts of Interest
References
- Shobha, R.; Siddaraju, C.; Suresh, K.R.; Niranjan, H.B. Mechanical property evaluation of heat treated insitu Al- TiB2 composite after severe plastic deformation. Mater. Today Proc. 2018, 5, 2534–2540. [Google Scholar] [CrossRef]
- Ma, Z.Y.; Li, J.H.; Li, S.X.; Ning, X.G.; Lu, Y.X.; Bi, J. Property-microstructure correlation in in situ formed Al2O3, TiB2 and Al3Ti mixture-reinforced aluminium composites. J. Mater. Sci. 1996, 31, 741–747. [Google Scholar] [CrossRef]
- Sadeghi, B.; Shamanian, M.; Cavaliere, P.; Ashrafizadeh, F. Effect of processing parameters on the microstructural and mechanical properties of aluminum–carbon nanotube composites produced by spark plasma sintering. Int. J. Mater. Res. 2018, 109, 900–909. [Google Scholar] [CrossRef]
- Takata, N.; Ishihara, M.; Suzuki, A.; Kobashi, M. Microstructure and strength of a novel heat-resistant aluminum alloy strengthened by T-Al6Mg11Zn11 phase at elevated temperatures. Mater. Sci. Eng. A 2019, 739, 62–70. [Google Scholar] [CrossRef]
- Zhou, H.; Qian, Z.; Zhou, M.; Liu, X.; Li, Y.; Zhang, X. Synergistic balance of strength and corrosion resistance in Al–Mg–Er alloys. Acta Metall. Sin. (Engl. Lett.) 2020, 33, 659–670. [Google Scholar] [CrossRef]
- Nakatsuka, S.; Ishihara, M.; Takata, N.; Suzuki, A.; Kobashi, M. Tensile Properties of a Heat-Resistant Aluminium Alloy Strengthened by T-Al6Mg11Zn11 Intermetallic Phase. MRS Adv. 2019, 4, 1485–1490. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Pathak, L.C.; Ray, A.K.; Das, G.; Verma, P.K.; Kumar, M.; Ghosh, R.N. Microstucture, tensile strength and wear behaviour of Al-Sc alloy. Mater. Sci. Eng. A 2004, 383, 374–380. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Gao, H.Y.; Wang, Y.F.; Wang, J.; De Sun, B.; Gu, S.W.; You, W.R. Effects of y addition on microstructure and properties of Al-Zr alloys. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2014, 24, 2239–2243. [Google Scholar] [CrossRef]
- Barlow, I.C.; Jones, H.; Rainforth, W.M. Evolution of microstructure and hardening, and the role of Al3Ti coarsening, during extended thermal treatment in mechanically alloyed Al-Ti-O based materials. Acta Mater. 2001, 49, 1209–1224. [Google Scholar] [CrossRef]
- Guoxian, L.; Zhichao, L.; Erde, W. Thermal stability and mechanical properties of mechanically alloyed Al-10Ti alloy. J. Mater. Sci. 1996, 31, 901–904. [Google Scholar] [CrossRef]
- Prosviryakov, A.S.; Shcherbachev, K.D.; Tabachkova, N.Y. Investigation of nanostructured Al-10 wt.% Zr material prepared by ball milling for high temperature applications. Mater. Charact. 2017, 123, 173–177. [Google Scholar] [CrossRef]
- Prosviryakov, A.S.; Shcherbachev, K.D.; Tabachkova, N.Y. Microstructural characterization of mechanically alloyed Al-Cu-Mn alloy with zirconium. Mater. Sci. Eng. A 2015, 623, 109–113. [Google Scholar] [CrossRef]
- Knipling, K.E.; Dunand, D.C.; Seidman, D.N. Precipitation evolution in Al-Zr and Al-Zr-Ti alloys during aging at 450–600 °C. Acta Mater. 2008, 56, 1182–1195. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, G.; Chen, X.G. Effects of two-step homogenization on precipitation behavior of Al3Zr dispersoids and recrystallization resistance in 7150 aluminum alloy. Mater. Charact. 2015, 102, 122–130. [Google Scholar] [CrossRef]
- Souza, P.H.L.; Do Vale Quaresma, J.M.; De Oliveira, C.A.S. Precipitation evolution and modeling of growth kinetics of L12-structured Al3Zr particles in Al-0.22Zr and Al-0.32Zr (wt.%) alloys isothermally aged. Mater. Res. 2017, 20, 1600–1613. [Google Scholar] [CrossRef] [Green Version]
- Janghorban, A.; Antoni-Zdziobek, A.; Lomello-Tafin, M.; Antion, C.; Mazingue, T.; Pisch, A. Phase equilibria in the aluminium-rich side of the Al-Zr system. J. Therm. Anal. Calorim. 2013, 114, 1015–1020. [Google Scholar] [CrossRef]
- Ostwald, W. Über die vermeintliche Isomerie des roten und gelben Quecksilberoxyds und die Oberflächenspannung fester Körper. Z. Phys. Chem. 2017, 34, 495–503. [Google Scholar] [CrossRef]
- Mursalat, M.; Schoenitz, M.; Dreizin, E.L. Composite Al∙Ti powders prepared by high-energy milling with different process controls agents. Adv. Powder Technol. 2019, 30, 1319–1328. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, X. Effects of Process Control Agents on the Mechanical Alloying Behavior of Nb-Ti-Si Based Alloy. Mater. Trans. 2018, 59, 528–537. [Google Scholar] [CrossRef] [Green Version]
- Cipolloni, G.; Pellizzari, M.; Molinari, A.; Hebda, M.; Zadra, M. Contamination during the high-energy milling of atomized copper powder and its effects on spark plasma sintering. Powder Technol. 2015, 275, 51–59. [Google Scholar] [CrossRef]
- Srinivasarao, B.; Suryanarayana, C.; Oh-Ishi, K.; Hono, K. Microstructure and mechanical properties of Al-Zr nanocomposite materials. Mater. Sci. Eng. A 2009, 518, 100–107. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Zadorozhnyy, V.Y.; Ketov, S.V.; Wang, Z.; Tsarkov, A.A.; Greer, A.L. On room-temperature quasi-elastic mechanical behaviour of bulk metallic glasses. Acta Mater. 2017, 129, 343–351. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, W.; Wei, X.; Ding, Y.; Shen, X.; Liu, W. Effect of Ti addition on mechanical properties and corrosion resistance of Ni-free Zr-based bulk metallic glasses for potential biomedical applications. J. Alloys Compd. 2020, 815, 152636. [Google Scholar] [CrossRef]
- Zhang, L.; Li, B.; Wu, H.; Wang, W.; Zhai, S.; Xu, J.; Niu, Z.; Wang, Y. Microstructure and property characterization of Al-based composites reinforced with CuZrAl particles fabricated by mechanical alloying and spark plasma sintering. Adv. Powder Technol. 2018, 29, 1695–1702. [Google Scholar] [CrossRef]
- Shi, H.; Li, Z.; Hu, Z.; Ding, Y.; Tang, T.; Shen, X. Enhancing strength and plasticity of Zr-based bulk metallic glasses by Zr partially substituted Fe and isothermal annealing. J. Non-Cryst. Solids 2020, 543. [Google Scholar] [CrossRef]
- Dutkiewicz, J.; Rogal, Ł.; Wajda, W.; Kukuła-Kurzyniec, A.; Coddet, C.; Dembinski, L. Aluminum Matrix Composites Strengthened with CuZrAgAl Amorphous Atomized Powder Particles. J. Mater. Eng. Perform. 2015, 24, 2266–2273. [Google Scholar] [CrossRef]
- Dudina, D.V.; Georgarakis, K.; Aljerf, M.; Li, Y.; Braccini, M.; Yavari, A.R.; Inoue, A. Cu-based metallic glass particle additions to significantly improve overall compressive properties of an Al alloy. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1551–1557. [Google Scholar] [CrossRef]
- Scudino, S.; Liu, G.; Prashanth, K.G.; Bartusch, B.; Surreddi, K.B.; Murty, B.S.; Eckert, J. Mechanical properties of Al-based metal matrix composites reinforced with Zr-based glassy particles produced by powder metallurgy. Acta Mater. 2009, 57, 2029–2039. [Google Scholar] [CrossRef]
- Tan, W.; Huang, L.; Li, S.; He, J. Microstructure and Mechanical Properties of Zr–Al–Ni–Cu Metallic Glassy Particles Reinforced 7056 Al Alloy Matrix Composites Obtained by Spark Plasma Sintering. Adv. Eng. Mater. 2019, 21, 1801267. [Google Scholar] [CrossRef]
- Janovszky, D. Influence of the Oxide and Ethanol Surface Layer on Phase Transformation of Al-Based Nanocomposite Powders under High-Energy Milling. Materials 2019, 12, 1305. [Google Scholar] [CrossRef] [Green Version]
- Triwikantoro; Toma, D.; Meuris, M.; Köster, U. Oxidation of Zr-based metallic glasses in air. J. Non-Cryst. Solids 1999, 250, 719–723. [Google Scholar] [CrossRef]
- Köster, U.; Jastrow, L. Oxidation of Zr-based metallic glasses and nanocrystalline alloys. Mater. Sci. Eng. A 2007, 449, 57–62. [Google Scholar] [CrossRef]
- Kematick, R.J.; Franzen, H.F. Journal of Solid State Chemistry. J. Solid State Chem. 1984, 54, 226–234. [Google Scholar] [CrossRef]
- Kaptay, G. A new equation for the temperature dependence of the excess Gibbs energy of solution phases. Calphad 2004, 28, 115–124. [Google Scholar] [CrossRef]
- Tamim, R.; Mahdouk, K. Thermodynamic reassessment of the Al–Zr binary system. J. Therm. Anal. Calorim. 2018, 131, 1187–1200. [Google Scholar] [CrossRef]
- Laik, A.; Bhanumurthy, K.; Kale, G.B. Intermetallics in the Zr-Al diffusion zone. Intermetallics 2004, 12, 69–74. [Google Scholar] [CrossRef]
- Alexander, W.B.; Slifkin, L.M. Diffusion of solutes in aluminum and dilute aluminum alloys. Phys. Rev. B 1970, 1, 3274–3282. [Google Scholar] [CrossRef]
- Marumo, T.; Fujikawa, S.; Hirano, K. Diffusion of zirconium in aluminum. J. Jpn. Inst. Light Met. 1973, 23, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Beke, D.L. Diffusion in Nonequilibrium Materials. Key Eng. Mater. 1995, 103, 51–78. [Google Scholar] [CrossRef]
- Mantina, M.; Wang, Y.; Chen, L.Q.; Liu, Z.K.; Wolverton, C. First principles impurity diffusion coefficients. Acta Mater. 2009, 57, 4102–4108. [Google Scholar] [CrossRef]
- Kidson, G.V.; Miller, G.D. A study of the interdiffusion of aluminum and zirconium. J. Nucl. Mater. 1964, 12, 61–69. [Google Scholar] [CrossRef]
- Wagner, C. The evaluation of data obtained with diffusion couples of binary single-phase and multiphase systems. Acta Metall. 1969, 17, 99–107. [Google Scholar] [CrossRef]
- Mehta, A.; Dickson, J.; Newell, R.; Keiser, D.D.; Sohn, Y. Interdiffusion and Reaction Between Al and Zr in the Temperature Range of 425 to 475 °C. J. Phase Equilibria Diffus. 2019, 40, 482–494. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, M.; Wu, X.; Jia, Z.; Wang, R.; Li, W.; Liu, Q. The structural stability, mechanical properties and stacking fault energy of Al3Zr precipitates in Al-Cu-Zr alloys: HRTEM observations and first-principles calculations. J. Alloy. Compd. 2016, 681, 96–108. [Google Scholar] [CrossRef]
- Jin, N.; Zhang, H.; Han, Y.; Wu, W.; Chen, J. Hot deformation behavior of 7150 aluminum alloy during compression at elevated temperature. Mater. Charact. 2009, 60, 530–536. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, H.; Han, Y.; Wu, W.; Chen, J. Hot deformation behavior of 2026 aluminum alloy during compression at elevated temperature. Mater. Sci. Eng. A 2010, 527, 485–490. [Google Scholar] [CrossRef]
- Choi, S.-H.; Sung, S.-Y.; Choi, H.-J.; Sohn, Y.-H.; Han, B.-S.; Lee, K.-A. High Temperature Tensile Deformation Behavior of New Heat Resistant Aluminum Alloy. Procedia Eng. 2011, 10, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, S.; Okuhira, T.; Mitsuhara, M.; Nakashima, H.; Kusui, J.; Adachi, M. Effect of Fe Addition on Heat-Resistant Aluminum Alloys Produced by Selective Laser Melting. Metals 2019, 9, 468. [Google Scholar] [CrossRef] [Green Version]













| Phases | Experiment | A1 | A2 | A2 | |||
|---|---|---|---|---|---|---|---|
| BPR:10/1 | BPR:10/1 | Sintered | |||||
| Volume of Ethanol | 0 | 1 cm3 | |||||
| Milling Time, h | 0 | 15 | 30 | 15 | 30 | ||
| Al Fm_3m | wt.% | 90.00 | 82.24 | 83.84 | 83.4 | 87.68 | 75.5 |
| CuZr2 I4/mmm | wt.% | 7.06 | 8.61 | 7.46 | - | 0.28 | 3.8 |
| CuZr(Ag, Al) Fm_3m | wt.% | 0.74 | 1.92 | 2.10 | 0.30 | 0.34 | - |
| CuZr Fm_3m | wt.% | - | - | - | 0.3 | 0.35 | 0.6 |
| Al0.8Ag3.2 Fm_3m | wt.% | 0.85 | 5.17 | 4.93 | 3.7 | 0.35 | 2.4 |
| Al3Zr I4/mmm | wt.% | - | - | - | 1.8 | - | 2.4 |
| AgO | wt.% | - | - | - | 0.1 | - | 0.8 |
| Al2FeO4 I4_1/amd | wt.% | - | - | - | 0.1 | - | - |
| γ-Al2O3 Fd_3m | wt.% | - | - | - | 0.3 | - | 1.2 |
| α-Iron Im_3m | wt.% | 0.15 | 2.07 | 1.67 | - | - | - |
| CuAl2 | wt.% | - | - | - | - | - | 2.3 |
| amorphous | wt.% | 0.12 | - | - | 10.00 | 11.00 | 11.0 |
| Rwp | - | - | - | 39.07 | - | 13.49 | 20.07 |
| GOF | - | - | - | 3.33 | - | 1.15 | 1.07 |
| Matrix | Diffusing Atom | Atomic Radius, nm | D0, m2/s | E, kJ/mol | D25 °C, m2/sec | D400 °C, m2/sec | Reference |
|---|---|---|---|---|---|---|---|
| Al | Cu | 0.1278 | 4.37 × 10−6 | 120.6 | 3.17 × 10−27 | 1.90 × 10−15 | [40] |
| - | Cu | 0.1278 | 8.4 × 10−5 | 136.0 | 1.22 × 10−28 | 2.34 × 10−15 | [24] |
| - | Ag | 0.1445 | 0.13 × 10−6 | 117.0 | 4.03 × 10−28 | 1.08 × 10−15 | [37] |
| - | Zr | 0.1603 | 7.28 × 10−4 | 241.8 | 3.00 × 10−46 | 1.24 × 10−22 | [38] |
| Cu | Al | 0.1432 | 4.8 × 10−6 | 165.5 | 4.69 × 10−35 | 6.85 × 10−19 | [24] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janovszky, D. Strengthening of Nanocrystalline Al with Al3Zr Core-Shell Structure. Metals 2020, 10, 1144. https://doi.org/10.3390/met10091144
Janovszky D. Strengthening of Nanocrystalline Al with Al3Zr Core-Shell Structure. Metals. 2020; 10(9):1144. https://doi.org/10.3390/met10091144
Chicago/Turabian StyleJanovszky, Dora. 2020. "Strengthening of Nanocrystalline Al with Al3Zr Core-Shell Structure" Metals 10, no. 9: 1144. https://doi.org/10.3390/met10091144
APA StyleJanovszky, D. (2020). Strengthening of Nanocrystalline Al with Al3Zr Core-Shell Structure. Metals, 10(9), 1144. https://doi.org/10.3390/met10091144