Role of Small Addition of Sc and Zr in Clustering and Precipitation Phenomena Induced in AA7075
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
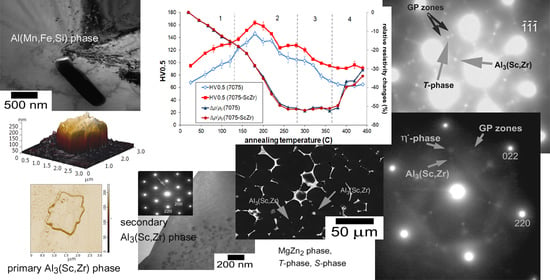
3.1. Heat-Treated (Initial) State of the Alloys
3.2. Natural Ageing of the Heat-Treated Alloys
3.3. Phase Development during Isochronal Annealing
3.4. Thermal Properties of the Alloys
4. Conclusions
- The microstructure of the initial (heat-treated) state of the alloys is very complex. The observation proved that the eutectic phases consist of four types at ones: MgZn2 phase, Al2CuMg phase (S-phase), Al2Zn3Mg3/Mg32(Al,Cu,Zn)49 phase (T-phase) and primary cubic λ-Al(Mn,Fe,Si) phase. In addition, two types of non-eutectic particles in the alloys with Sc–Zr addition are present: Primary incoherent Al3(Sc,Zr) particles with square and polygonal shapes and secondary coherent Al3(Sc,Zr) particles.
- The Sc,Zr-containing alloys cannot be completely homogenized due to these elements. However, for the 7xxx-based system, a heat treatment above ~440 °C is sufficient (unless eutectic phase is considered). It is not even possible to observe the difference in characterization of the properties after the high-temperature annealing at 470 °C/60 min and 470 °C/240 min, respectively.
- Microhardness values reflect the Sc–Zr addition in the 7075-ScZr alloy. Strengthening is caused by the presence of the primary/secondary Al3(Sc,Zr)-phase particles. Positive influence on corrosion properties is also caused by the Sc–Zr addition.
- Single and/or multiple Zn- or Mg-solutes and/or Zn,Mg(-co)-clusters developed during quenching immediately after high temperature treatment in the alloys. These solute (co-)clusters further evolve in the course of natural ageing. This process causes a significant increase in microhardness and electrical resistivity values. A long term natural ageing leads to a coarsening of the solute (co-)clusters into bigger objects (probably precursors of the GP zones).
- Addition of Sc and Zr has a slight negative influence on the concentration of solute (co-)clusters. Si, Cu or Mg solutes are most probably bound to Sc and Zr solutes and/or to the Al3Sc(Zr)-phase particles. However, in general it can be said that the co-presence of the Sc- and Zr- elements have only a little effect on the phase transformations of the Al–Zn–Mg–Cu system.
- Formation of the η’/η-phase is slightly suppressed in the alloys after natural ageing. The dissolution of the precursors of the GP zones/GP zones is shifted to higher temperatures depending on the time of natural ageing.
- The apparent activation energy values of the observed thermal processes were calculated as: Formation of the precursors of the GP zones/GP zones: ~82 kJ/mol, dissolution of the (co-)clusters/GP zones: ~102 kJ/mol, formation of the metastable η’-phase: ~112 kJ/mol, formation of the stable T-phase: ~133 kJ/mol, formation of the stable η-phase: ~125 kJ/mol.
- A combination of both precipitation sequences of the Al–Zn–Mg–Cu-based system was observed in the studied alloys.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toropova, L.S.; Eskin, D.G.; Kharakterova, M.L.; Dobatkina, T.V. Advanced Aluminium Alloys Containing Scandium—Structure and Properties, 1st ed.; Gordon and Breach Science Publisher: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Roskill—Commodity Research, Consulting & Events. Available online: http://www.roskill.com/ (accessed on 2 April 2020).
- Schuman, S.; Friedrich, F. The use of magnesium in cars—Today and in future. In Magnesium Alloys and Their Applications; Mordike, B.L., Kainer, K.U., Eds.; Werkstoff-Informationsgesselschaft: Frankfurt, Germany, 1998. [Google Scholar]
- Jones, M.J.; Humphreys, F. Interaction of recrystallization and precipitation: The effect of Al3Sc on the recrystallization behaviour of deformed aluminium. Acta Mater. 2003, 51, 2149–2159. [Google Scholar] [CrossRef]
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Ghosh, A.; Ghosh, M. Microstructure and texture development of 7075 alloy during homogenisation. Philos. Mag. A Mater Sci. 2018, 98, 1470–1490. [Google Scholar] [CrossRef]
- Wen, K.; Xiong, B.; Zhang, Y.; Li, Z.; Li, X.; Yan, L.; Yan, H.; Liu, H. Measurement and Theoretical Calculation Confirm the Improvement of T7651 Aging State Influenced Precipitation Characteristics on Fatigue Crack Propagation Resistance in an Al–Zn–Mg–Cu Alloy. Met. Mater. Int. 2019, 1–17. [Google Scholar] [CrossRef]
- Yuan, B.; Guo, M.; Wu, Y.; Zhang, J.; Zhuang, L.-Z.; Lavernia, E.J. Influence of treatment pathways on the precipitation behaviors of Al-Mg-Si-Cu-(Zn)-Mn alloys. J. Alloys Compd. 2019, 797, 26–38. [Google Scholar] [CrossRef]
- Mondal, C.; Mukhopadhyay, A. On the nature of T(Al2Mg3Zn3) and S(Al2CuMg) phases present in as-cast and annealed 7055 aluminum alloy. Mater. Sci. Eng. A 2005, 391, 367–376. [Google Scholar] [CrossRef]
- Zhang, Y.; Pelliccia, D.; Milkereit, B.; Kirby, N.; Starink, M.J.; Rometsch, P. Analysis of age hardening precipitates of Al-Zn-Mg-Cu alloys in a wide range of quenching rates using small angle X-ray scattering. Mater. Des. 2018, 142, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Mostafapoor, S.; Malekan, M.; Emamy, M. Thermal analysis study on the grain refinement of Al–15Zn–2.5Mg–2.5Cu alloy. J. Therm. Anal. Calorim. 2017, 127, 1941–1952. [Google Scholar] [CrossRef]
- Shu, S.; De Luca, A.; Dunand, D.C.; Seidman, D.N. Effects of W micro-additions on precipitation kinetics and mechanical properties of an Al–Mn–Mo–Si–Zr–Sc–Er alloy. Mater. Sci. Eng. A 2020, 140550. [Google Scholar] [CrossRef]
- Vo, N.Q.; Seidman, D.N.; Dunand, D.C. Effect of Si micro-addition on creep resistance of a dilute Al-Sc-Zr-Er alloy. Mater. Sci. Eng. A 2018, 734, 27–33. [Google Scholar] [CrossRef]
- Li, H.; Gao, Z.; Yin, H.; Jiang, H.; Su, X.; Bin, J. Effects of Er and Zr additions on precipitation and recrystallization of pure aluminum. Scr. Mater. 2013, 68, 59–62. [Google Scholar] [CrossRef]
- Kodetová, V.; Vlach, M.; Kudrnová, H.; Leibner, M.; Málek, J.; Cieslar, M.; Bajtošová, L.; Harcuba, P.; Neubert, V. Annealing effects in commercial aluminium hot-rolled 7075(–Sc–Zr) alloys. J. Therm. Anal. Calorim. 2020, 142, 1613–1623. [Google Scholar] [CrossRef]
- Glerum, J.A.; Kenel, C.; Sun, T.; Dunand, D.C. Synthesis of precipitation-strengthened Al-Sc, Al-Zr and Al-Sc-Zr alloys via selective laser melting of elemental powder blends. Addit. Manuf. 2020, 36, 101461. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, T.; He, C.; Ding, J.; Liu, E.; Shi, C.; Li, J.; Zhao, N. Evolution of microstructure and properties of Al–Zn–Mg–Cu–Sc–Zr alloy during aging treatment. J. Alloys Compd. 2016, 658, 946–951. [Google Scholar] [CrossRef]
- Emani, S.V.; Benedyk, J.; Nash, P.; Chen, D. Double aging and thermomechanical heat treatment of AA7075 aluminum alloy extrusions. J. Mater. Sci. 2009, 44, 6384–6391. [Google Scholar] [CrossRef]
- Vlach, M.; Čížek, J.; Kodetová, V.; Kekule, T.; Lukáč, F.; Cieslar, M.; Kudrnová, H.; Bajtošová, L.; Leibner, M.; Harcuba, P.; et al. Annealing Effects in Cast Commercial Aluminium Al–Mg–Zn–Cu(–Sc–Zr) Alloys. Met. Mater. Int. 2019, 1–10. [Google Scholar] [CrossRef]
- Vlach, M.; Čížek, J.; Kodetova, V.; Leibner, M.; Cieslar, M.; Harcuba, P.; Bajtosova, L.; Kudrnova, H.; Vlasak, T.; Neubert, V.; et al. Phase transformations in novel hot-deformed Al–Zn–Mg–Cu–Si–Mn–Fe(–Sc–Zr) alloys. Mater. Des. 2020, 193, 108821. [Google Scholar] [CrossRef]
- Rometsch, P.A.; Zhang, Y.; Knight, S. Heat treatment of 7xxx series aluminium alloys—Some recent developments. Trans. Nonferr. Met. Soc. China 2014, 24, 2003–2017. [Google Scholar] [CrossRef]
- Rout, P.K.; Ghosh, M.; Ghosh, K. Microstructural, mechanical and electrochemical behaviour of a 7017 Al–Zn–Mg alloy of different tempers. Mater. Charact. 2015, 104, 49–60. [Google Scholar] [CrossRef]
- Feng, C.; Shou, W.-B.; Liu, H.; Yi, D.; Feng, Y.-R. Microstructure and mechanical properties of high strength Al–Zn–Mg–Cu alloys used for oil drill pipes. Trans. Nonferr. Met. Soc. China 2015, 25, 3515–3522. [Google Scholar] [CrossRef]
- Starink, M.; Wang, S. A model for the yield strength of overaged Al–Zn–Mg–Cu alloys. Acta Mater. 2003, 51, 5131–5150. [Google Scholar] [CrossRef] [Green Version]
- Vlach, M.; Kodetová, V.; Smola, B.; Čízek, J.; Kekule, T.; Cieslar, M.; Kudrnová, H.; Bajtošová, L.; Leibner, M.; Procházka, I. Characterization of phase development in commercial Al-Zn-Mg(-Mn,Fe) alloy with and without Sc,Zr-addition. Met. Mater. 2018, 56, 367–377. [Google Scholar] [CrossRef] [Green Version]
- Priya, P.; Johnson, D.R.; Krane, M.J. Precipitation during cooling of 7XXX aluminum alloys. Comput. Mater. Sci. 2017, 139, 273–284. [Google Scholar] [CrossRef]
- Shu, W.; Hou, L.; Zhang, C.; Zhang, F.; Liu, J.; Liu, J.; Zhuang, L.; Zhang, J. Tailored Mg and Cu contents affecting the microstructures and mechanical properties of high-strength Al–Zn–Mg–Cu alloys. Mater. Sci. Eng. A 2016, 657, 269–283. [Google Scholar] [CrossRef]
- Yang, X.; Chen, J.; Liu, J.; Qin, F.; Xie, J.; Wu, C. A high-strength AlZnMg alloy hardened by the T-phase precipitates. J. Alloys Compd. 2014, 610, 69–73. [Google Scholar] [CrossRef]
- Zhao, Y.; Liao, X.; Jin, Z.; Valiev, R.; Zhu, Y.T. Microstructures and mechanical properties of ultrafine grained 7075 Al alloy processed by ECAP and their evolutions during annealing. Acta Mater. 2004, 52, 4589–4599. [Google Scholar] [CrossRef]
- Li, Z.; Xiong, B.; Zhang, Y.; Zhu, B.; Wang, F.; Liu, H. Investigation of microstructural evolution and mechanical properties during two-step ageing treatment at 115 and 160 °C in an Al–Zn–Mg–Cu alloy pre-stretched thick plate. Mater. Charact. 2008, 59, 278–282. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, D.; Ding, Q.; Zhang, J.; Zhuang, L.-Z. Solute clustering and precipitation of Al-5.1Mg-0.15Cu-xZn alloy. Mater. Sci. Eng. A 2019, 759, 465–478. [Google Scholar] [CrossRef]
- Berg, L.; Gjønnes, J.; Hansen, V.; Li, X.; Knutson-Wedel, M.; Waterloo, G.; Schryvers, D.; Wallenberg, L. GP-zones in Al–Zn–Mg alloys and their role in artificial aging. Acta Mater. 2001, 49, 3443–3451. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Chen, J.; Wan, C.; Fang, L.; Liu, P.; Wu, C. Relationship Between the Strengthening Effect and the Morphology of Precipitates in Al–7.4Zn–1.7Mg–2.0Cu Alloy. Acta Met. Sin. (Engl. Lett.) 2014, 27, 1070–1077. [Google Scholar] [CrossRef] [Green Version]
- Booth-Morrison, C.; Seidman, D.N.; Dunand, D.C. Effect of Er additions on ambient and high-temperature strength of precipitation-strengthened Al–Zr–Sc–Si alloys. Acta Mater. 2012, 60, 3643–3654. [Google Scholar] [CrossRef]
- Michi, R.A.; Toinin, J.P.; Farkoosh, A.R.; Seidman, D.N.; Dunand, D.C. Effects of Zn and Cr additions on precipitation and creep behavior of a dilute Al–Zr–Er–Si alloy. Acta Mater. 2019, 181, 249–261. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Yi, D.; Wang, B.; Wang, H. Comparative study of Sc and Er addition on microstructure, mechanical properties, and electrical conductivity of Al-0.2Zr-based alloy cables. Mater. Charact. 2018, 145, 126–134. [Google Scholar] [CrossRef]
- Yu, T.; Li, B.; Medjahed, A.; Hou, L.; Wu, R.; Zhang, J.; Sun, J.; Zhang, M. Impeding effect of the Al3(Er,Zr,Li) particles on planar slip and intergranular fracture mechanism of Al-3Li-1Cu-0.1Zr-X alloys. Mater. Charact. 2019, 147, 146–154. [Google Scholar] [CrossRef]
- Zhang, C.; Yin, D.; Jiang, Y.; Wang, Y. Precipitation of L12-phase nano-particles in dilute Al-Er-Zr alloys from the first-principles. Comput. Mater. Sci. 2019, 162, 171–177. [Google Scholar] [CrossRef]
- Bochvar, N.; Rybalchenko, O.; Leonova, N.; Tabachkova, N.; Rokhlin, L. Effect of cold plastic deformation and subsequent aging on the strength properties of Al-Mg2Si alloys with combined (Sc + Zr) and (Sc + Hf) additions. J. Alloys Compd. 2020, 821, 153426. [Google Scholar] [CrossRef]
- Verdier, M.; Janecek, M.; Brechet, Y.; Guyot, P. Microstructural evolution during recovery in Al–2.5%Mg alloys. Mater. Sci. Eng. A 1998, 248, 187–197. [Google Scholar] [CrossRef]
- Neubert, V.; Smola, B.; Stulíková, I.; Bakkar, A.; Reuter, J. Microstructure, mechanical properties and corrosion behaviour of dilute Al–Sc–Zr alloy prepared by powder metallurgy. Mater. Sci. Eng. A 2007, 464, 358–364. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.-T.; Wang, J.-Y. Influence of Preaging Treatment on Bake-Hardening Response and Electrochemical Corrosion Behavior of High Strength Al-Zn-Mg-Cu-Zr Alloy. Metals 2019, 9, 895. [Google Scholar] [CrossRef] [Green Version]
- Bečvář, F.; Čížek, J.; Procházka, I.; Janotová, J. The asset of ultra-fast digitizers for positron-lifetime spectroscopy. Nucl. Instrum. Methods Phys. Sect. A 2005, 539, 372–385. [Google Scholar] [CrossRef]
- Vlach, M.; Čížek, J.; Melikhova, O.; Stulíková, I.; Smola, B.; Kekule, T.; Kudrnová, H.; Gemma, R.; Neubert, V. Early Stages of Precipitation Process in Al-(Mn-)Sc-Zr Alloy Characterized by Positron Annihilation. Met. Mater. Trans. A 2015, 46, 1556–1564. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Zhang, Z.; Li, M.; Pan, D.; Su, H.; Du, X.; Li, P.; Wu, Y. Effect of Sc on microstructure and mechanical properties of as-cast Al–Mg alloys. Mater. Des. 2016, 90, 1077–1084. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Z.; Li, M.; Pan, D.; Su, H.; Du, X.; Li, P.; Wu, Y. Formation of the eutecticum with multilayer structure during solidification in as-cast Al–Mg alloy containing a high level of Sc. Mater. Lett. 2016, 164, 19–22. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Z.; Li, M.; Pan, D.; Su, H.; Du, X.; Li, P.; Wu, Y. Correlative characterization of primary particles formed in as-cast Al-Mg alloy containing a high level of Sc. Mater. Charact. 2016, 118, 85–91. [Google Scholar] [CrossRef]
- Li, J.; Wiessner, M.; Albu, M.; Wurster, S.; Sartory, B.; Hofer, F.; Schumacher, P. Correlative characterization of primary Al3(Sc,Zr) phase in an Al–Zn–Mg based alloy. Mater. Charact. 2015, 102, 62–70. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, X.; Zhang, X.; Liu, M.; Liang, Z.; Leyvraz, D.; Banhart, J. Natural ageing clustering under different quenching conditions in an Al-Mg-Si alloy. Scr. Mater. 2021, 190, 179–182. [Google Scholar] [CrossRef]
- Liu, S.; Li, C.; Han, S.; Deng, Y.; Zhang, X. Effect of natural aging on quench-induced inhomogeneity of microstructure and hardness in high strength 7055 aluminum alloy. J. Alloys Compd. 2015, 625, 34–43. [Google Scholar] [CrossRef]
- Liu, M.; Čížek, J.; Chang, C.S.; Banhart, J. Early stages of solute clustering in an Al–Mg–Si alloy. Acta Mater. 2015, 91, 355–364. [Google Scholar] [CrossRef]
- Chinh, N.Q.; Lendvai, J.; Ping, D.; Hono, K. The effect of Cu on mechanical and precipitation properties of Al–Zn–Mg alloys. J. Alloys Compd. 2004, 378, 52–60. [Google Scholar] [CrossRef]
- Dellah, M.; Bournane, M.; Ragab, K.A.; Sadaoui, Y.; Sirenko, A. Early decomposition of supersaturated solid solutions of Al–Zn–Mg casting alloys. Mater. Des. 2013, 50, 606–612. [Google Scholar] [CrossRef]
- Royset, J.; Ryum, N. Scandium in aluminium alloys. Int. Mater. Rev. 2005, 50, 19–44. [Google Scholar] [CrossRef]
- Olafsson, P.; Sandström, R.; Karlsson, A. Comparison of experimental, calculated and observed values for electrical and thermal conductivity of aluminium alloys. J. Mater. Sci. 1997, 32, 4383–4390. [Google Scholar] [CrossRef]
- Skoko, Ž.; Popović, S.; Štefanić, G. Microstructure of Al–Zn and Zn–Al Alloys. Croat. Chem. Acta 2009, 82, 405–420. [Google Scholar]
- Khalfallah, A.; Raho, A.A.; Amzert, S.; Djemli, A. Precipitation kinetics of GP zones, metastable η′ phase and equilibrium η phase in Al−5.46wt.%Zn−1.67wt.%Mg alloy. Trans. Nonferr. Met. Soc. China 2019, 29, 233–241. [Google Scholar] [CrossRef]
- Hou, S.; Liu, P.; Zhang, D.; Zhang, J.; Zhuang, L.-Z. Precipitation hardening behavior and microstructure evolution of Al–5.1 Mg–0.15Cu alloy with 3.0Zn (wt%) addition. J. Mater. Sci. 2017, 53, 3846–3861. [Google Scholar] [CrossRef]
- Ganiev, I.N. High-Temperature and Electrochemical Corrosion of Aluminum-Scandium Alloys. Protect. Met. 1995, 31, 543–546. [Google Scholar]
- Kharina, G.V.; Kochergin, V.P. Corrosion–Electrochemical Behavior of Al–Zn–rem Alloys in the Presence of Polyvanadate-ions. Prot. Met. 2001, 37, 575–579. [Google Scholar] [CrossRef]
- Yoshioka, H.; Habazaki, H.; Kawashima, A.; Asami, K.; Hashimoto, K. The corrosion behavior of sputter-deposited AlZr alloys in 1 M HCl solution. Corros. Sci. 1992, 33, 425–436. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, Q.; Li, M.; Huang, X.; Li, B. Effect of Sc and Zr additions on corrosion behaviour of Al–Zn–Mg–Cu alloys. J. Alloys Compd. 2014, 612, 42–50. [Google Scholar] [CrossRef]
- Ghosh, K.; Gao, N.; Starink, M. Characterisation of high pressure torsion processed 7150 Al–Zn–Mg–Cu alloy. Mater. Sci. Eng. A 2012, 552, 164–171. [Google Scholar] [CrossRef]
- Ghosh, K.; Gao, N. Determination of kinetic parameters from calorimetric study of solid state reactions in 7150 Al-Zn-Mg alloy. Trans. Nonferr. Met. Soc. China 2011, 21, 1199–1209. [Google Scholar] [CrossRef]
- Belov, N.A.; Korotkova, N.; Akopyan, T.; Tsydenov, K. Simultaneous Increase of Electrical Conductivity and Hardness of Al–1.5 wt.% Mn Alloy by Addition of 1.5 wt.% Cu and 0.5 wt.% Zr. Metals 2019, 9, 1246. [Google Scholar] [CrossRef] [Green Version]
- Belov, N.A.; Korotkova, N.O.; Akopyan, T.; Pesin, A. Phase composition and mechanical properties of Al–1.5%Cu–1.5%Mn–0.35%Zr(Fe,Si) wire alloy. J. Alloys Compd. 2019, 782, 735–746. [Google Scholar] [CrossRef]
- Vlach, M.; Stulíková, I.; Smola, B.; Císařová, H.; Piešová, J.; Daniš, S.; Gemma, R.; Málek, J.; Tanprayoon, D.; Neubert, V. Phase transformations in non-isothermally annealed as-cast and cold-rolled AlMnScZr alloys. Int. J. Mater. Res. 2012, 103, 814–820. [Google Scholar] [CrossRef]
- Myhr, O. Modelling of the age hardening behaviour of Al–Mg–Si alloys. Acta Mater. 2001, 49, 65–75. [Google Scholar] [CrossRef]
- Myhr, O.R.; Grong, Ø.; Schäfer, C. An Extended Age-Hardening Model for Al-Mg-Si Alloys Incorporating the Room-Temperature Storage and Cold Deformation Process Stages. Met. Mater. Trans. A 2015, 46, 6018–6039. [Google Scholar] [CrossRef]
- Starink, M. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Antonione, C.; Marino, F.; Riontino, G.; Abis, S.; Di Russo, E. An evaluation of AA 7012 microstructure by differential scanning calorimetry. Mater. Chem. Phys. 1988, 20, 13–25. [Google Scholar] [CrossRef]
- Afify, N.; Gaber, A.-F.; Abbady, G. Fine Scale Precipitates in Al-Mg-Zn Alloys after Various Aging Temperatures. Mater. Sci. Appl. 2011, 2, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Abis, S.; Riontino, G. A resistivity study of 7012 Al-Zn-Mg alloy commercial tempers. Mater. Lett. 1987, 5, 442–445. [Google Scholar] [CrossRef]
- Fan, X.; Jiang, D.; Meng, Q.; Zhong, L. The microstructural evolution of an Al–Zn–Mg–Cu alloy during homogenization. Mater. Lett. 2006, 60, 1475–1479. [Google Scholar] [CrossRef]
- Styles, M.; Hutchinson, C.; Chen, Y.; Deschamps, A.; Bastow, T. The coexistence of two S (Al2CuMg) phases in Al–Cu–Mg alloys. Acta Mater. 2012, 60, 6940–6951. [Google Scholar] [CrossRef]














| Alloy | Mg | Zn | Cu | Mn | Si | Fe | Sc | Zr |
|---|---|---|---|---|---|---|---|---|
| 7075 | 3.44 | 2.76 | 0.79 | 0.21 | 0.22 | 0.18 | - | - |
| 7075-ScZr | 3.41 | 2.72 | 0.77 | 0.26 | 0.24 | 0.23 | 0.14 | 0.06 |
| State of the Alloys | Heat Treatment |
|---|---|
| HT | 470 °C/60 min |
| 470 °C/240 min | 470 °C/240 min |
| HT + NA | 470 °C/60 min + natural ageing for 3500 h |
| HT + NA15000 | 470 °C/60 min + natural ageing for 15,000 h |
| 470 °C/240 min + NA | 470 °C/240 min + natural ageing for 3500 h |
| Alloy | Heat Treatment | HV0.5 | ρ (nΩ·m) | |||||
|---|---|---|---|---|---|---|---|---|
| Initial State | NA 33 h | NA 450 h | NA 3500 h | Initial State | NA 33 h | NA 3500 h | ||
| 7075 | HT state | 70 ± 3 | 117 ± 2 | 134 ± 3 | 145 ± 2 | 27 ± 1 | 35 ± 1 | 36 ± 1 |
| 7075 | 470 °C/240 min | 66 ± 6 | 115 ± 2 | 134 ± 2 | 144 ± 4 | - | - | - |
| 7075-ScZr | HT state | 95 ± 4 | 139 ± 5 | 155 ± 6 | 168 ± 3 | 26 ± 1 | 33 ± 1 | 34 ± 1 |
| 7075-ScZr | 470 °C/240 min | 94 ± 4 | 138 ± 5 | 154 ± 4 | 167 ± 2 | - | - | - |
| Alloy | Icorr (µA/cm2) | WL (g/m2/d) | CR (mm/a) | Ecorr (mV) |
|---|---|---|---|---|
| 7075 | 2.96 | ~0.238 | ~0.0322 | −1154 |
| 7075-ScZr | 2.23 | ~0.180 | ~0.0243 | −1125 |
| Effect/Alloy | 1 °C/min | 2 °C/min | 5 °C/min | 10 °C/min | 20 °C/min |
|---|---|---|---|---|---|
| Effect A (7075 HT) | 58 | 63 | 72 | 83 | 90 |
| Effect A (7075-ScZr HT) | 58 | 63 | 72 | 84 | 91 |
| Effect B (7075 HT + NA) | 106 | 110 | 118 | 126 | 141 |
| Effect B (7075-ScZr HT + NA) | 104 | 110 | 118 | 127 | 142 |
| Effect C (7075 HT) | 165 | 177 | 191 | 201 | 209 |
| Effect C (7075-ScZr HT) | 165 | 178 | 189 | 198 | - |
| Effect D (7075 HT) | 208 | 219 | 231 | 239 | 255 |
| Effect D (7075-ScZr HT) | 207 | 219 | 230 | 241 | 255 |
| Effect D (7075 HT + NA) | 207 | 217 | 231 | 242 | 249 |
| Effect D (7075-ScZr HT + NA) | 208 | 216 | 229 | 239 | 250 |
| Effect E (7075 HT) | 237 | 255 | 263 | 277 | 295 |
| Effect E (7075-ScZr HT) | 237 | 254 | 265 | 276 | 294 |
| Effect E (7075 HT + NA) | 240 | 254 | 267 | 277 | 298 |
| Effect E (7075-ScZr HT + NA) | 238 | 256 | 265 | 277 | 292 |
| Sample | Effect (Process) | Activation Energy Q (kJ/mol) |
|---|---|---|
| 7075 HT | A (GP zones formation) | 84 ± 6 |
| C (η’-phase precipitation) | 111 ± 6 | |
| D (T-phase precipitation) | 130 ± 11 | |
| E (η-phase precipitation) | 122 ± 9 | |
| 7075-ScZr HT | A (GP zones formation) | 81 ± 5 |
| C (η’-phase precipitation) | 113 ± 10 | |
| D (T-phase precipitation) | 127 ± 10 | |
| E (η-phase precipitation) | 124 ± 10 | |
| 7075 HT + NA | B (GP zones dissolution) | 103 ± 13 |
| D (T-phase precipitation) | 136 ± 5 | |
| E (η-phase precipitation) | 122 ± 12 | |
| 7075-ScZr HT + NA | B (GP zones dissolution) | 100 ± 11 |
| D (T-phase precipitation) | 137 ± 5 | |
| E (η-phase precipitation) | 130 ± 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlach, M.; Kodetova, V.; Cizek, J.; Leibner, M.; Kekule, T.; Lukáč, F.; Cieslar, M.; Bajtošová, L.; Kudrnová, H.; Sima, V.; et al. Role of Small Addition of Sc and Zr in Clustering and Precipitation Phenomena Induced in AA7075. Metals 2021, 11, 8. https://doi.org/10.3390/met11010008
Vlach M, Kodetova V, Cizek J, Leibner M, Kekule T, Lukáč F, Cieslar M, Bajtošová L, Kudrnová H, Sima V, et al. Role of Small Addition of Sc and Zr in Clustering and Precipitation Phenomena Induced in AA7075. Metals. 2021; 11(1):8. https://doi.org/10.3390/met11010008
Chicago/Turabian StyleVlach, Martin, Veronika Kodetova, Jakub Cizek, Michal Leibner, Tomáš Kekule, František Lukáč, Miroslav Cieslar, Lucia Bajtošová, Hana Kudrnová, Vladimir Sima, and et al. 2021. "Role of Small Addition of Sc and Zr in Clustering and Precipitation Phenomena Induced in AA7075" Metals 11, no. 1: 8. https://doi.org/10.3390/met11010008
APA StyleVlach, M., Kodetova, V., Cizek, J., Leibner, M., Kekule, T., Lukáč, F., Cieslar, M., Bajtošová, L., Kudrnová, H., Sima, V., Zikmund, S., Cernoskova, E., Kutalek, P., Neubert, V. -D., & Neubert, V. (2021). Role of Small Addition of Sc and Zr in Clustering and Precipitation Phenomena Induced in AA7075. Metals, 11(1), 8. https://doi.org/10.3390/met11010008