Susceptibility to Pitting Corrosion of Ti-CP2, Ti-6Al-2Sn-4Zr-2Mo, and Ti-6Al-4V Alloys for Aeronautical Applications
Abstract
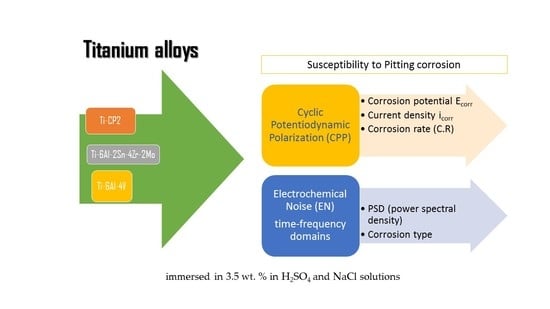
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Microstructural Characterization
2.3. Electrochemical Techniques
3. Results
3.1. OM—SEM Microstructural Analysis
3.2. Electrochemical Test
3.2.1. Cyclic Potentiodynamic Polarization (CPP)
3.2.2. Power Spectral Density Analysis (PSD)
3.3. SEM Analysis
4. Discussion
5. Conclusions
- Titanium alloys did not show susceptibility to pitting corrosion as observed in cyclic potentiodynamic polarization.
- The electrochemical results indicated that Ti-alloys developed passive layers in NaCl and H2SO4 solutions, but the passive layer is more stable in H2SO4 than the NaCl solution. Instability is related to Cl− ions that avoids the proper layer growth.
- The passive layer was unstable for Ti-6Al-2Sn-4Zr-2Mo in the NaCl solution because Cl− ions penetrated the oxide surface, being an unstable layer. CPP and PSD results confirm this with pseudopassivation and fluctuations, respectively. Further, molybdenum in the β phase facility the instability of the passive layer.
- Corrosion rates increase in the H2SO4 solution due to passive layer dissolution and the metal surface was exposed to the electrolyte.
- Ti-6Al-4V had higher corrosion rates; this did attribute to phases differences. β phase induced the development of vanadium oxide, which provoked an oxide layer with bigger pores so Cl− and OH− ions could penetrate the alloy. The difference in the oxide layer and OH− concentration induced cathodic loops in Ti-6Al-4V.
- SEM observations presented diffusion of Cl− and Na+ on the material surface. For the H2SO4 solution, samples showed oxygen presence, but presence increase in Ti CP2, because this alloy did not have a phase difference, was only in α.
- Given the enormous industrial importance of this type of titanium alloy and by obtaining a better understanding of their corrosion behavior, we recognize that the use of powerful electrochemical techniques would be of great benefit.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mouritz, P.A. Introduction to Aerospace Materials, 1st ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 202–223. [Google Scholar]
- Gialanella, S.; Malandruccolo, A. Aerospace Alloys, 1st ed.; Springer: Cham, Switzerland, 2020; pp. 129–189. [Google Scholar]
- Peters, M.; Hemptenmacher, J.; Kumpfert, J.; Leyens, C. Structure and Properties of Titanium and Titanium Alloys, 1st ed.; Wiley-VCH Verkag GmbG & Co. KGaA: Cologne, Germany, 2003; pp. 4–27. [Google Scholar]
- Barington, N.; Black, M. Aerospace Materials and Manufacturing Processes at the Millennium. In Aerospace Materials; Cantor, B., Assender, H., Grant, P., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 3–15. [Google Scholar]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Olguín-Coca, J.; López-Léon, L.D.; Flores-De los Rios, J.P.; Almeraya-Calderón, F. Electrochemical Noise Analysis of the Corrosion of Titanium Alloys in NaCl and H2SO4 Solutions. Metals 2021, 11, 105. [Google Scholar] [CrossRef]
- Koshal, D. Metal Casting and Moulding Processes. In Manufacturing Engineer’s Reference Book; Koshal, D., Ed.; Butterworth Heinemann: Brighton, UK, 1993; pp. 1–23. [Google Scholar]
- Yang, X.; Liu, C.R. Machining titanium and its alloys. Mach. Learn. 1998, 3, 107–139. [Google Scholar] [CrossRef]
- Sha, W.; Malinov, S. Titanium Alloys: Modelling of Microstructure, Properties and Applications, 1st ed.; Malinov, S., Ed.; CRC Press: Oxford, UK, 2009; pp. 237–255. [Google Scholar]
- Ahmad, Z. Principles of Corrosion Engineering and Corrosion Control, 1st ed.; Ahmad, Z., Ed.; Butterworth-Heinemann: London, UK, 2006; pp. 120–270. [Google Scholar]
- Moiseyev, V.N. Titanium Alloys Russian Aircraft and Aerospace Applications, 1st ed.; Fridlayander, J.N., Eskin, D.G., Eds.; Taylor & Francis CRC Press: Boca Raton, FL, USA, 2005; pp. 164–173. [Google Scholar]
- Klimecka-Tatar, D. Electrochemical characteristics of titanium for dental implants in case of the electroless surface modification. Arch. Metall. Mater. 2016, 61, 923–926. [Google Scholar] [CrossRef] [Green Version]
- Klimecka-Tatar, D.; Borkowski, S.; Sygut, P. The kinetics of Ti-1Al-1Mn alloy thermal oxidation and characteristic of oxide layer. Arch. Metall. Mater. 2015, 60, 735–738. [Google Scholar] [CrossRef] [Green Version]
- Jagielska-Wiaderek, K.; Bala, H.; Wierzchon, T. Corrosion depth profiles of nitrided titanium alloy in acidified sulphate solution. Cent. Eur. J. Chem. 2013, 11, 2005–2011. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Leyland, A.; Bi, Q.; Matthews, A. Corrosion resistance of multi-layerded plasma-assisted physical vapour deposition TiN and CrN coatings. Surf. Coat. Technol. 2001, 141, 164–173. [Google Scholar] [CrossRef]
- Balla, A.; Marcu, C.; Axante, D.; Borodi, G.; Lazar, D. Catalytic reduction of sulfuric acid to sulfur dioxide. Cent. Eur. J. Chem. 2012, 10, 1817–1823. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Dong, X.; Li, W.; Feng, W.; Xu, Y. Effect of solution and aging treatments on corrosion performance of laser solid formed Ti-6Al-4V alloy in a 3.5 wt. % NaCl solution. J. Mater. Res. Technol. 2020, 9, 1559–1568. [Google Scholar] [CrossRef]
- Casillas, N.; Charlebois, S.; Smyrl, W.H.; White, H.S. Pitting Corrosion of Titanium. J. Electrochem. Soc. 1994, 141, 636. [Google Scholar] [CrossRef]
- Du, X.; Yang, Q.S.; Chen, Y.; Zhang, X.Z. Galvanic Corrosion behavior of copper/titanium galvanic couple in artificial seawater. Trans. Nonferrous Met. Soc. China 2014, 24, 570–581. [Google Scholar] [CrossRef]
- Fu, Y.; Zhou, F.; Wang, Q.; Zhang, M.; Zhou, Z. Electrochemical tribocorrosion performances of CrMoSiCN coating on Ti-6Al-4V titanium alloy in artificial seawater. Corros. Sci. 2020, 165, 108385. [Google Scholar] [CrossRef]
- Caha, I.; Alves, A.C.; Chirico, C.; Tsipas, S.A.; Rodrigues, I.R.; Pinto, A.M.P.; Grandini, C.R.; Rocha, L.A.; Gordo, E.; Toptan, F. Interactions between wear and corrosion cast and sintered Ti-12Nb alloy in comparison with the commercial Ti-6Al-4V alloy. Corros. Sci. 2020, 176, 108925. [Google Scholar] [CrossRef]
- Chen, W.Q.; Zhang, S.M.; Qiu, J. Surface analysis and corrosion behavior of pure titanium under fluoride exposure. J. Prosthet. Dent. 2020, 124, 239.e1–239.e8. [Google Scholar] [CrossRef]
- Dubent, S.; Mazard, A. Characterization and corrosion behaviour of grade 2 titanium used in electrolyzers for hydrogen production. Int. J. Hydrogen Energy 2019, 44, 15622–15633. [Google Scholar] [CrossRef]
- Lara-Banda, M.; Gaona-Tiburcio, C.; Zambrano-Robledo, P.; Delgado-E, M.; Cabral-Miramontes, J.A.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Estupiñan-López, F.; Chacón-Nava, J.G.; Almeraya-Calderón, F. Alternative to Nitric Acid Passivation of 15-5 and 17-4PH Stainless Steel Using Electrochemical Techniques. Materials 2020, 13, 2836. [Google Scholar] [CrossRef]
- Nady, H.; El-Rabiei, M.M.; Samy, M. Corrosion behavior and electrochemical properties of carbon steel, commercial pure titanium copper and copper-aluminum-nickel alloy in 3.5% sodium chloride containing sulfide ions. Egypt. J. Pet. 2017, 26, 79–94. [Google Scholar] [CrossRef] [Green Version]
- Magdy, A.M.; Ibrahim, D.; Yoshimura, P.M. The electrochemical behavior and characterization of the anodic oxide film formed on titanium in NaOH solutions. J. Solid State Electrochem. 2002, 6, 341–350. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Y.; Huo, W.; Zhang, W.; Zhao, Y.; Zhang, Y. Electrochemical corrosion characteristics and biocompatibility of nanostructured titanium for implants. Appl. Surf. Sci. 2018, 434, 63–72. [Google Scholar] [CrossRef]
- Hu, P.; Song, R.; Li, X.J.; Deng, J.; Chen, Z.Y.; Li, Q.W.; Wang, K.; Cao, W.; Liu, D.; Yu, H. Influence of concentrations of chloride ions on electrochemical corrosion behavior of titanium-zirconium-molybdenum alloy. J. Alloys Compd. 2017, 367–372. [Google Scholar] [CrossRef]
- Jelliti, S.; Richard, C.; Retraint, D.; Roland, T.; Chemkhi, M.; Demangel, C. Effect of surface nanocrystallization on the corrosion behavior of Ti-6Al-4V titanium alloy. Sur. Coat. Technol. 2013, 224, 82–87. [Google Scholar] [CrossRef]
- Ji, R.; Wang, B.; Jin, H.; Liu, Y.; Cheng, W.; Cai, B.; Li, X. Removing loose oxide layer and producing dense α-phase layer simultaneously to improve corrosion resistance of Ti-6Al-4V titanium alloy by coupling electrical pulse and ultrasonic treatment. Surf. Coat. Technol. 2020, 384, 125329. [Google Scholar] [CrossRef]
- Risking, J.; Khentov, A. Electrocorrosion and Protection of Metals, 2nd ed.; Risking, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 225–248. [Google Scholar]
- Kuphasuk, C.; Oshida, Y.; Andres, C.J.; Hovijitra, S.T.; Barco, M.T.; Brown, D.T. Electrochemical corrosion of titanium and titanium-based alloys. J. Prothet. Dent. 2001, 85, 195–202. [Google Scholar] [CrossRef]
- Pink, H.; Rui, S.; Kuaishe, W.; Fan, Y.; Boliang, H.; Zhen-Lu, C.; Qinwe, L.; Weicheng, C.; Dongxin, L.; Lei, G.; et al. Electrochemical Corrosion Behavior of Titanium-Zirconium-Molybdenum Alloy. Rare Metal Mat. Eng. 2017, 46, 1225–1230. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Ahmed, T.; Rack, H.J. Phase transformations in Ti-Nb-Ta and Ti-Nb-Ta-Zr alloys. J. Mater. Sci. 2000, 35, 1805–1811. [Google Scholar] [CrossRef]
- Soltis, J. Passivity breakdown, pit initiation and propagation of pits in metallic materials—Review. Corr. Sci. 2015, 90, 5–22. [Google Scholar] [CrossRef]
- Natishan, P.M.; O’Grady, W.R. Chloride Ion Interactions with Oxide-Covered Aluminum Leading to Pitting Corrosion: A Review. J. Electrochem. Soc. 2014, 161, C421–C432. [Google Scholar] [CrossRef]
- Al-Saadi, S.; Yi, Y.; Cho, P.; Jang, C.; Beeley, P. Passivity breakdown of 316L stainless steel during potentiodynamic polarization in NaCl solution. Corros. Sci. 2016, 720–727. [Google Scholar] [CrossRef]
- Seo, D.; Lee, J.B. Effects of competitive anion adsorption (Br- or Cl-) and semiconducting properties of the passive films on the corrosion behavior of the additively manufactured Ti-6Al-4V alloys. Corros. Sci. 2020, 173, 108789. [Google Scholar] [CrossRef]
- Galván-Martinez, R.; Orozco-Cruz, R.; Torres-Sanchez, R.; Martinez, E.A. Corrosion study of the X52 steel immersed in seawater with a corrosion inhibitor using a rotating cylinder electrode. Mater. Corros. 2010, 61, 872–876. [Google Scholar] [CrossRef]
- Corral-Higuera, R.; Arredondo-Rea, P.; Neri-Flores, M.A.; Gómez-Soberón, J.M.; Almaral-Sánchez, J.L.; Castorena-González, J.C.; Almeraya-Calderón, F. Chloride ion penetrability and Corrosion Behavior of Steel in Concrete with Sustainability Characteristics. Int. J. Electrochem. Sci. 2011, 6, 958–970. [Google Scholar]
- Noel, J.J.; Shoesmith, D.W. Ebrahimi, N. Corrosion of Titanium, and Its Alloys, 1st ed.; Wandelt, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 193–199. [Google Scholar]
- Galván-Martínez, R.; Cabrera-de la Cruz, D.; Contreras, A.; Orozco-Cruz, R. A novel experimental arrangement for corrosión study of X60 pipeline steel weldments at turbulent flow conditions. Corros. Eng. Sci. Technol. 2016, 5, 1–8. [Google Scholar] [CrossRef]
- Gaona-Tiburcio, C.; Aguilar, L.M.R.; Zambrano-Robledo, P.; Estupiñán-López, F.; Cabral-Miramontes, J.A.; Nieves-Mendoza, D.; Castillo-González, E.; Almeraya-Calderón, F. Electrochemical Noise Analysis of Nickel Based Superalloys in Acid Solutions. Int. J. Electrochem. Sci. 2014, 9, 523–533. [Google Scholar]
- Estupiñán-López, H.F.; Almeraya-Calderón, F.; Bautista Margulis, G.R.; Baltazar Zamora, M.A.; Martínez-Villafañe, A.; Uruchurtu, C.J.; Gaona-Tiburcio, C. Transient Analysis of Electrochemical Noise for 316 and Duplex 2205 Stainless Steels Under Pitting Corrosion. Int. J. Electrochem. Sci. 2011, 6, 1785–1796. [Google Scholar]
- Contreras, A.; Salazar, M.; Carmona, A.; Galván-Martínez, R. Electrochemical Noise for Detection of Stress Corrosion Cracking of Low Carbon Steel Exposed to Synthetic Soil Solution. Mater. Res. 2017, 20, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sedriks, A.J.; Green, J.A.; Novak, D.L. Electrochemical behavior of Ti-Ni alloys in acidic chloride solutions. Corrosion 1972, 28, 137–142. [Google Scholar] [CrossRef]
- Tafel, J. Über die polarization bei kathodischer wasserstoffentwicklung. Z. Phys. Chem. 1905, 641–712. [Google Scholar] [CrossRef]
- Wagner, C.; Traud, W. Über die Deutung von Korrosionsvorgängen durch Überlagerung von elektrochemischen Teilvorgängen und über die Potentialbildung an Mischelektroden, Z. Elektrochem; Springer: Berlin/Heidelberg, Germany, 1951; pp. 391–454. [Google Scholar] [CrossRef]
- Butler, J.A.V. Studies in heterogeneous equilibria. Part II.—The kinetic interpretation on the Nernst theory of electromotive force. Trans. Faraday Soc. 1924, 729–733. [Google Scholar] [CrossRef]
- Erdey-Gruz, T.; Volmer, M. Zur Theorie der Wasserstoff Überspannung. Z. Phys. Chem. 1930, 150, 203–213. [Google Scholar] [CrossRef]
- Esmailzadeh, S.; Aliofkhazraei, M.; Sarlak, H. Interpretation of Cyclic Potentiodynamic Polarization Test Results for Study of Corrosion Behavior of Metals: A Review. Prot. Met. Phys. Chem. Sulf. 2018, 54, 976–989. [Google Scholar] [CrossRef]
- Silverman, D.C. Tutorial on Cyclic Potentiodynamic Polarization Technique; NACE Corrosion/98; NACE International: Houston, TX, USA, 1998. [Google Scholar]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Beck, T.R.; Blackburn, M.J.; Smyrl, W.H. Stress Corrosion Cracking of Titanium Alloys: Electrochemical kinetics, SCC Studies with Ti:8-1-1, SCC and Polarization Curves in Molten Salts, Liquid Metal Embrittlement, and SCC Studies with other Titanium Alloys. In Solid State Physics Laboratory; Boeing Scientific Research Laboratories: Seattle, WA, USA, 1969. [Google Scholar]
- Cabral-Miramontes, J.A.; Gaona-Tiburcio, C.; Almeraya-Calderón, F.; Estupiñan-Lopez, H.F.; Pedraza-Basulto, G.; Poblano-Salas, C. Parameter Studies on High-Velocity Oxy-Fuel Spraying of CoNiCrAlY Coatings Used in the Aeronautical Industry. Int. J. Corros. 2014, 1–8. [Google Scholar] [CrossRef]
- Kearns, J.R.; Eden, D.A.; Yaffe, M.R.; Fahey, J.V.; Reichert, D.L.; Silverman, D.C. ASTM Standardization of Electrochemical Noise Measurement. Electrochemical Noise Measurement for Corrosion Applications; Kearns, J.R., Scully, J.R., Roberge, P.R., Reirchert, D.L., Dawson, L., Eds.; ASTM International, Materials Park: Novelty, OH, USA, 1996; pp. 446–471. [Google Scholar]
- Botana, P.J.; Bárcena, M.M.; Villero, Á.A. Ruido Electroquímico: Métodos de Análisis; Septem Ediciones: Cadiz, Spain, 2002; pp. 50–70. [Google Scholar]
- Legat, A.; Dolecek, V. Corrosion Monitoring System Based on Measurement and Analysis of electrochemical Noise. Corrosion 1995, 51, 295–300. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Nishikata, A.; Sakairi, M.; Fushimi, K. Electrochemistry for Corrosion Fundamentals; Springer Singapore: Singapore, 2018; pp. 140–156. [Google Scholar]
- ASTM E3-95. Standard Practice for Preparation of Metallographic Specimens; ASTM International: West Conshohocken, PA, USA, 1995. [Google Scholar]
- ASTM E407-07. Standard Practice for Microetching Metals and Alloys; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- ASTM G5—14e1. Standard Reference Test Method for Making Potentiodynamic Anodic Polarization Measurements; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- ASTM G61-86. Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements for Localized Corrosion Susceptibility of Iron, Nickel or Cobalt-Based Alloys; ASTM International: West Conshohocken, PA, USA, 1998. [Google Scholar]
- ASTM G199-09. Standard Guide for Electrochemical Noise Measurement; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- Nabhani, M.; Razavi, R.S.; Barekat, M. Corrosion study of laser cladded Ti-6Al-4V alloy in different corrosive environments. Eng. Fail. Anal. 2019, 97, 234–241. [Google Scholar] [CrossRef]
- Bocchetta, P.; Chen, L.-Y.; Tardelli, J.D.C.; Reis, A.C.d.; Almeraya-Calderón, F.; Leo, P. Passive Layers and Corrosion Resistance of Biomedical Ti-6Al-4V and β-Ti Alloys. Coatings 2021, 11, 487. [Google Scholar] [CrossRef]
- Froes, F.; Quian, M. Titanium for Consumer Applications. Real World Use of Titanium; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 27–65. [Google Scholar] [CrossRef]
- Seah, K.H.W.; Thampuran, R.; Teoh, S.H. The influence of pore morphology on corrosion. Corros. Sci. 1998, 40, 547–556. [Google Scholar] [CrossRef]
- Adamek, G.; Pałka, K.; Jakubowicz, J. Corrosion properties of Ti scaffolds prepared with sucrose as a space holder. Solid State Phenom. 2015, 227, 519–522. [Google Scholar] [CrossRef]
- Dabrowski, B.; Kaminski, J.; Swieszkowski, W.; Kurzydlowski, K.J. Porous titanium scaffolds for biomedical applications: Corrosion resistance and structure investigation. Mater. Sci. Forum 2011, 674, 41–46. [Google Scholar] [CrossRef]
- Chen, X.; Fu, Q.; Jin, Y.; Li, M.; Yang, R.; Cui, X.; Gong, M. In vitro studying corrosion behavior of porous titanium coating in dynamic electrolyte. Mater. Sci. Eng. C 2017, 70, 1071–1075. [Google Scholar] [CrossRef]
- Prando, D.; Nicolis, D.; Pedeferri, M.; Ormellese, M. Pitting corrosion on anodized titanium: Effect of halades. Mater. Corros. 2018, 69, 1–6. [Google Scholar] [CrossRef]
- Maldonado-Bandala, E.; Jiménez-Quero, V.; Olguin-Coca, J.; Lizarraga, L.G.; Baltazar-Zamora, M.A.; Ortiz-C, A.; Almeraya, C.F.; Zambrano, R.P.; Gaona-Tiburcio, C. Electrochemical Characterization of Modified Concretes with Sugar Cane Bagasse Ash. Int. J. Electrochem. Sci. 2011, 6, 4915–4926. [Google Scholar]
- Wang, Z.B.; Hu, H.X.; Zheng, Y.G. Synergistic effects of fluoride and chloride on general corrosion behavior of AISI 316 stainless steel and pure titanium in H2SO4 solutions. Corros. Sci. 2018, 130, 203–217. [Google Scholar] [CrossRef]
- Beck, T.R.; Blackburn, M.J. Stress corrosion cracking of titanium alloys. AIAA J. 1968, 6, 326–332. [Google Scholar] [CrossRef]
- Gao, K.W.; Chu, W.Y.; Gu, B.; Zhang, T.C.; Qiao, L.J. In-Situ Transmission Electron Microscopic Observation of Corrosion-Enhanced Dislocation Emission and Crack Initiation of Stress Corrosion. Corrosion 2000, 56, 515–522. [Google Scholar] [CrossRef]
- De la Garza-Ramos, M.A.; Estupiñan-Lopez, F.H.; Gaona-Tiburcio, C.; Beltrán-Novelo, L.G.; Zambrano-Robledo, P.; Cabral-Miramontes, J.; Almeraya-Calderón, F. Electrochemical Behavior of Ti6Al4V Alloy Used in Dental Implants Immersed in Streptococcus gordonii and Fusobacterium nucleatum Solutions. Materials 2020, 13, 4185. [Google Scholar] [CrossRef]
- Almeraya-Calderón, F.; Montoya-R, M.; Garza Montes de Oca, N.; Castorena, J.H.; Estupiñan, F.; Cabral, J.; Maldonado, E.; Gaona-Tiburcio, C. Corrosion behavior of multilayer Coatings deposited by PVD Inconel 718 in Chloride and Sulphuric Acid solutions. Int. J. Electrochem. Sci. 2019, 14, 9596–9609. [Google Scholar] [CrossRef]
- Takahashi, K.; Kagawa, T.; Tanaka, K.; Kihira, H.; Ushioda, K. Reduction of Contact Resistance on Titanium Sheet Surfaces by Formation of Titanium Carbide and Nitride, and its Stability in Sulfuric Acid Aqueous Solution. ISIJ Int. 2019, 59, 1621–1631. [Google Scholar] [CrossRef] [Green Version]
- Cabral-Miramontes, J.; Gaona-Tiburcio, C.; Estupinán-López, F.; Lara-Banda, M.; Zambrano-Robledo, P.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Chacón-Nava, J.; Almeraya-Calderón, F. Corrosion Resistance of Hard Coat Anodized AA 6061 in Citric–Sulfuric Solutions. Coatings 2020, 10, 601. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.A.; Barceinas-Sánchez, J.D.O.; Poblano-Salas, C.A.; Pedraza-Basulto, G.K.; Nieves-Mendoza, D.; Zambrano-Robledo, P.C.; Almeraya-Calderón, F.; Chacón-Nava, J.G. Corrosion Behavior of AISI 409Nb Stainless Steel Manufactured by Powder Metallurgy Exposed in H2SO4 and NaCl Solutions. Int. J. Electrochem. Sci. 2013, 8, 564–577. [Google Scholar]
- Pellegrini-Cervantes, M.J.; Almeraya-Calderon, F.; Borunda-Terrazas, A.; Bautista-Margulis, R.G.; Chacón-Nava, J.G.; Fajardo-San-Miguel, G.; Almaral-Sanchez, J.L.; Barrios-Durstewitz, C.; Martinez-Villafañe, A. Corrosion Resistance, Porosity and Strength of lended Portland Cement Mortar Containing Rice Husk Ash And Nano-SiO2. Int. J. Electrochem. Sci. 2013, 8, 10697–10710. [Google Scholar]
- Doll, P.W.; Wolf, M.; Weichert, M.; Ahrens, R.; Spindler, B.; Guber, A.E. Nanostructuring of Titanium by Anodic Oxidation with Sulfuric and Hydrofluoric Acid. Curr. Direc. Biomed. Eng. 2018, 4, 641–644. [Google Scholar] [CrossRef]
- Krýsa, J.; Mráz, R.; Sousar, I. Corrosion rate of titanium in H2SO4. Mat. Chem. Phys. 1997, 48, 64–67. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, E.; Xu, D.; Yang, Y.; Zhao, Y.; Zhang, Y.; Gu, T.; Yang, K.; Wang, F. Laboratory investigation of microbiologically influenced corrosion of 2205 duplex stainless steel by marine pseudomonas aeruginosa biofilm using electrochemical noise. Corrosion 2018, 143, 291. [Google Scholar] [CrossRef]
- Lee, J.W.; Osseo-Asare, K.; Pickering, H.W. Anodic dissolution of iron in ammoniacal ammonium carbonate solution. J. Electrochem. Soc. 1985, 132, 550. [Google Scholar] [CrossRef]
- Beavers, J.A.; Durr, C.L.; Thompson, N.G. Unique Interpretations of Potentiodynamic Polarization Technique. In NACE International: Corrosion/98, San Diego, California; paper 300; 1998. [Google Scholar]
- Ramgoal, T.; Schmutz, P.; Frankel, G.S. Electrochemical Behavior of Thin Film Analogs of Mg (Zn, Cu, Al)2. J. Electrochem. Soc. 2001, 148, B348. [Google Scholar] [CrossRef] [Green Version]
- Cabral-Miramontes, J.A.; Bastidas, D.M.; Baltazar, M.A.; Zambrano-Robledo, P.; Bastidas, J.M.; Almeraya-Calderón, F.M.; Gaona-Tiburcio, C. Corrosion behavior of Zn-TiO2, and Zn-ZnO Electrodeposited Coating in 3.5% NaCl Solution. Int. J. Electrochem. Sci. 2019, 14, 4226–4239. [Google Scholar] [CrossRef]
- Cabral Miramontes, J.A.; Barceinas Sánchez, J.D.O.; Almeraya Calderón, F.; Martínez Villafañe, A.; Chacón Nava, J.G. Effect of Boron Additions on Sintering and Densification of a Ferritic Stainless Steel. J. Mater. Eng. Perform. 2010, 19, 880–884. [Google Scholar] [CrossRef]
- Hernández, M.; Genescá, J.; Uruchurtu, J.; Barba, A. Correlation between electrochemical impedance and noise measurements of waterborne coatings. Corr. Sci. 2009, 499–510. [Google Scholar] [CrossRef]
- Uruchurtu, J.C.; Dawson, J.L. Noise Analysis of Pure Aluminum under Different Pitting Conditions. Corrosion 1987, 43, 19–26. [Google Scholar] [CrossRef]
- Lentka, L.; Smulko, J. Methods of trend removal in electrochemical noise data-overview. Measurement 2019, 131, 569–581. [Google Scholar] [CrossRef]
- Homborg, A.M.; Cottis, R.A.; Mol, J.M.C. An integrated approach in the time, frequency and time-frequency domain for the identification of corrosion using electrochemical noise. Electrochim. Acta 2016, 222, 627–640. [Google Scholar] [CrossRef]








| Alloys | Elements | ||
|---|---|---|---|
| Ti CP2 | Ti-6Al-2Sn-4Zr-2Mo | Ti-6Al-4V | |
| Fe | 0.038 ± 0.005 | – | 0.21 ± 0.01 |
| Al | – | 6.75 ± 0.20 | 7.14 ± 0.37 |
| V | – | – | 4.03 ± 0.08 |
| Zr | – | 4.18 ± 0.01 | – |
| Mo | – | 1.99 ± 0.008 | – |
| Sn | – | 2.08 ± 0.01 | – |
| Ti | 99.94 ± 0.005 | 84.65 ± 0.19 | 87.71 ± 0.36 |
| Alloys | Ecorr (V) | icorr (mA/cm2) | Ea-c (V) | Active-Passive Trans (V) | Hysteresis | Range Passive (V) | C.R. (mm/y) |
|---|---|---|---|---|---|---|---|
| NaCl | |||||||
| Ti-6Al-2Sn-4Zr-2Mo | −0.484 | 3.07 × 10−4 | N/A | – | Negative | – | 2.67 × 10−4 |
| Ti-6Al-4V | −0.394 | 13.1 × 10−3 | 0.230 | – | Negative | 1.104 | 5.90 × 10−4 |
| Ti CP2 | −0.490 | 10.3 × 10−3 | 0.286 | – | Negative | 1.025 | 5.26 × 10−4 |
| H2SO4 | |||||||
| Ti-6Al-2Sn-4Zr-2Mo | −0.589 | 10.0 × 10−3 | −0.297 | −0.477 | Negative | 1.739 | 1.06 × 10−2 |
| Ti-6Al-4V | −0.561 | 1.45 × 10−3 | −0.269 | – | Negative | 0.929 | 3.74 × 10−1 |
| Ti CP2 | −0.547 | 2.91 × 10−3 | 0.232 | −0.479 | Negative | 1.156 | 1.20 × 10−1 |
| Corrosion Type | dB(V)·Decade−1 | dB(A)·Decade−1 | ||
|---|---|---|---|---|
| Minimum | Maximum | Minimum | Maximum | |
| Uniform | 0 | −7 | 0 | −7 |
| Pitting | −20 | −25 | −7 | −14 |
| Passive | −15 | −25 | −1 | 1 |
| Ti-Alloys | Β (dB (V)) |
|---|---|
| NaCl | |
| Ti-6Al-2Sn-4Zr-2Mo | −20 |
| Ti-6Al-4V | −11 |
| Ti CP2 | −8 |
| H2SO4 | |
| Ti-6Al-2Sn-4Zr-2Mo | −7 |
| Ti-6Al-4V | −13 |
| Ti CP2 | −8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaquez-Muñoz, J.; Gaona-Tiburcio, C.; Lira-Martinez, A.; Zambrano-Robledo, P.; Maldonado-Bandala, E.; Samaniego-Gamez, O.; Nieves-Mendoza, D.; Olguin-Coca, J.; Estupiñan-Lopez, F.; Almeraya-Calderon, F. Susceptibility to Pitting Corrosion of Ti-CP2, Ti-6Al-2Sn-4Zr-2Mo, and Ti-6Al-4V Alloys for Aeronautical Applications. Metals 2021, 11, 1002. https://doi.org/10.3390/met11071002
Jaquez-Muñoz J, Gaona-Tiburcio C, Lira-Martinez A, Zambrano-Robledo P, Maldonado-Bandala E, Samaniego-Gamez O, Nieves-Mendoza D, Olguin-Coca J, Estupiñan-Lopez F, Almeraya-Calderon F. Susceptibility to Pitting Corrosion of Ti-CP2, Ti-6Al-2Sn-4Zr-2Mo, and Ti-6Al-4V Alloys for Aeronautical Applications. Metals. 2021; 11(7):1002. https://doi.org/10.3390/met11071002
Chicago/Turabian StyleJaquez-Muñoz, Jesus, Citlalli Gaona-Tiburcio, Alejandro Lira-Martinez, Patricia Zambrano-Robledo, Erick Maldonado-Bandala, Oliver Samaniego-Gamez, Demetrio Nieves-Mendoza, Javier Olguin-Coca, Francisco Estupiñan-Lopez, and Facundo Almeraya-Calderon. 2021. "Susceptibility to Pitting Corrosion of Ti-CP2, Ti-6Al-2Sn-4Zr-2Mo, and Ti-6Al-4V Alloys for Aeronautical Applications" Metals 11, no. 7: 1002. https://doi.org/10.3390/met11071002
APA StyleJaquez-Muñoz, J., Gaona-Tiburcio, C., Lira-Martinez, A., Zambrano-Robledo, P., Maldonado-Bandala, E., Samaniego-Gamez, O., Nieves-Mendoza, D., Olguin-Coca, J., Estupiñan-Lopez, F., & Almeraya-Calderon, F. (2021). Susceptibility to Pitting Corrosion of Ti-CP2, Ti-6Al-2Sn-4Zr-2Mo, and Ti-6Al-4V Alloys for Aeronautical Applications. Metals, 11(7), 1002. https://doi.org/10.3390/met11071002