Thermal Decomposition and Kinetics of Pentlandite-Bearing Ore Oxidation in the Air Atmosphere
Abstract
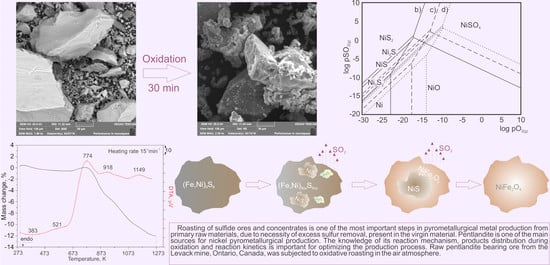
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Results of Chemical Analysis
3.2. X-ray Diffraction Results
3.3. SEM/EDS Analysis
3.4. The Results of Thermodynamic Analysis
3.5. The Results of Thermal Analysis
3.6. The Results of Kinetic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuck, P.H. Nickel Mineral Commodity Summaries; United States Geological Survey: Washington, DC, USA, 2010; Available online: https://s3-us-west-2.amazonaws.com/prd-wret/assets/palladium/production/mineral-pubs/nickel/mcs-2010-nicke.pdf (accessed on 12 February 2020).
- Kuck, P.H. Nickel Mineral Commodity Summaries; United States Geological Survey: Washington, DC, USA, 2015; Available online: https://s3-us-west-2.amazonaws.com/prd-wret/assets/palladium/production/mineral-pubs/nickel/mcs-2015-nicke.pdf (accessed on 12 February 2020).
- McRae, M.E. Nickel Mineral Commodity Summaries; United States Geological Survey: Washington, DC, USA, 2020. Available online: https://pubs.usgs.gov/periodicals/mcs2020/mcs2020-nickel.pdf (accessed on 12 February 2020).
- Betteridge, W. Nickel and Its Alloys; Ellis Horwood: Chichester, UK; Halsted Press: New York, NY, USA, 1984. [Google Scholar]
- Vracar, R.Z. Theory and Practice of Extraction of Non-Ferrous Metals/Teorija i Praksa Dobijanja Obojenih Metala; Serbian Association of Metallurgy Engineers: Belgrade, Serbia, 2010. [Google Scholar]
- Berndt, D. Maintenance-Free Batteries: Lead-Acid, Nickel/Cadmium, Nickel/Metal Hydride: A Handbook of Battery Technology, 2nd ed.; Research Studies Press: Baldock, UK, 1997. [Google Scholar]
- Linden, D.; Reddy, T.B. Handbook of Batteries, 3rd ed.; McGraw-Hill: New York, NY, USA, 2002. [Google Scholar]
- Gong, M.; Zhou, W.; Tsai, M.C.; Zhou, J.; Guan, M.; Lin, M.C.; Zhang, B.; Hu, Y.; Wang, D.Y.; Yang, J.; et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nat. Commun. 2014, 5, 4695. [Google Scholar] [CrossRef]
- Tasker, S.Z.; Standley, E.A.; Jamison, T.F. Recent advances in homogeneous nickel catalysis. Nature 2014, 509, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Konkena, B.; Junge Puring, K.; Sinev, I.; Piontek, S.; Khavryuchenko, O.; Dürholt, J.P.; Schmid, R.; Tüysüz, H.; Muhler, M.; Schuhmann, W.; et al. Pentlandite rocks as sustainable and stable efficient electrocatalysts for hydrogen generation. Nat. Commun. 2016, 7, 12269. [Google Scholar] [CrossRef]
- Sokić, M.; Ilić, I.; Živković, D.; Vučković, N. Investigation of mechanism and kinetics of chalcopyrite concentrate oxidation process. Metalurgija 2008, 47, 109–113. [Google Scholar]
- Mitovski, A.; Štrbac, N.; Mihajlović, I.; Sokić, M.; Stojanović, J. Thermodynamic and kinetic analysis of the polymetallic copper concentrate oxidation process. J. Therm. Anal. Calorim. 2014, 118, 1277–1285. [Google Scholar] [CrossRef]
- Crundwell, F.K.; Moats, M.S.; Ramachandran, V.; Robinson, T.G.; Davenport, W.G. Extractive Metallurgy of Nickel, Cobalt and Platinum-Group Metal, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Xia, F.; Pring, A.; Brugger, J. Understanding the mechanism and kinetics of pentlandite oxidation in extractive pyrometallurgy of nickel. Miner. Eng. 2012, 27–28, 11–19. [Google Scholar] [CrossRef]
- Goryachev, A.A.; Chernousenko, E.V.; Potapov, S.S.; Tsvetov, N.S.; Makarov, D.V. A Study of the Feasibility of Using Ammonium Sulfate in Copper—Nickel Ore Processing. Metals 2021, 11, 422. [Google Scholar] [CrossRef]
- Janjić, S.; Ristić, P. Mineralogy/Mineralogija; Scientific Book: Belgrade, Serbia, 1995. [Google Scholar]
- Diaz, C.M.; Landolt, C.A.; Vahed, A.; Warner, A.E.M.; Taylor, W. A review of nickel pyrometallurgical operations. JOM 1988, 40, 28–33. [Google Scholar] [CrossRef]
- Naldrett, A.J.; Craig, J.R.; Kullerud, G. The central portion of the Fe-Ni-S system and its bearing on pentlandite solution in iron-nickel sulfide ores. Econ. Geol. 1967, 62, 826–847. [Google Scholar] [CrossRef]
- Mishra, K.C.; Fleet, M.E. The chemical composition of synthetic and natural pentlandite assemblages. Econ. Geol. 1973, 68, 518–539. [Google Scholar] [CrossRef]
- Dunn, J.G. The oxidation of sulfide minerals. Thermochim. Acta 1997, 300, 127–139. [Google Scholar] [CrossRef]
- Mkhonto, P.P.; Chauke, H.R.; Ngoepe, P.E. Ab initio studies of O2 adsorption on (110) Nickel-rich pentlandite (Fe4Ni5S8) mineral surface. Minerals 2015, 5, 665–678. [Google Scholar] [CrossRef] [Green Version]
- Thornhill, P.G.; Pidgeon, L.M. Micrographic study of sulfide roasting. JOM 1957, 9, 989–995. [Google Scholar] [CrossRef]
- Ellingham, H.J.T. Reducibility of oxides and sulphides in metallurgical processes. J. Soc. Chem. Ind. 1944, 63, 125–133. [Google Scholar]
- Ashcroft, E.A. Process for the Treatment of Ores or Materials Containing Copper and/or Nickel. U.S. Patent 1,851,885, 29 March 1932. [Google Scholar]
- Tanabe, T.; Kawaguchi, K.; Asaki, Z.; Kondo, Y. Oxidation kinetics of dense pentlandite. Trans. JIM 1987, 28, 977–985. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, T.; Sturman, B.T. The oxidation of iron (II) sulphide. J. Therm. Anal. Calorim. 1975, 8, 329–337. [Google Scholar] [CrossRef]
- Dunn, J.G.; Kelly, C.E. A TG/MS and DTA study of the oxidation of nickel sulphide. J. Therm. Anal. Calorim. 1977, 12, 43–52. [Google Scholar] [CrossRef]
- Dunn, J.G.; Kelly, C.E. A TG/DTA/MS study of the oxidation of pentlandite. J. Therm. Anal. Calorim. 1980, 18, 147–154. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, J.; Deng, J.; Yu, R.; Xing, X. Oxidation behavior and mechanism of pentlandite at 973 K (700 °C) in air. Metall. Mater. Trans. B 2012, 43, 494–502. [Google Scholar] [CrossRef]
- Dunn, J.G.; Jayweera, S.A. Effect of heating rate on the TG curve during the oxidation of nickel sulfide concentrates. Thermochim. Acta 1983, 61, 313–317. [Google Scholar] [CrossRef]
- Pandher, R.; Utigard, T. Roasting of Nickel Concentrates. Metall. Mater. Trans. B 2010, 41, 780–789. [Google Scholar] [CrossRef]
- Yu, D.; Utigard, T.A. TG/DTA study on the oxidation of nickel concentrate. Thermochim. Acta 2012, 533, 56–65. [Google Scholar] [CrossRef]
- Hseu, Z.Y. Evaluating heavy metal contents in nine composts using four digestion methods. Bioresour. Technol. 2004, 95, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Shultz, J.I.; Bell, R.K.; Rains, T.C.; Menis, O. Methods of Analysis of NBS Clay Standards; National Bureau of Standards Special Publication 260-37; National Bureau of Standards: Washington, DC, USA, 1972; pp. 3–4. [Google Scholar]
- Borchard, H.J.; Daniels, F. The Application of Differential Thermal Analysis to the Study of Reaction Kinetics. J. Am. Chem. Soc. 1957, 79, 41–46. [Google Scholar] [CrossRef]
- Živković, D.; Živković, Ž. Problems in the Theory of Metallurgical Processes/Zbirka Zadataka iz Teorije Metlurških Procesa; University of Belgrade, Technical Faculty in Bor: Belgrade, Serbia, 2001. [Google Scholar]
- Roine, A. HSC Chemistry® v 9.0; Research Oy Center, Outotec: Pori, Finland, 2016. [Google Scholar]
- Shamsuddin, M. Roasting of Sulfide Minerals. In Physical Chemistry of Metallurgical Processes; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 39–71. [Google Scholar]
- Zhang, Y.; Li, Q.; Liu, X.; Xu, B.; Yang, Y.; Jiang, T. A Thermodynamic Analysis on the Roasting of Pyrite. Minerals 2019, 9, 220. [Google Scholar] [CrossRef] [Green Version]
- Smirnov, V.I.; Tihonov, A.I. Incineration of Copper Ores and Concentrates/Obţig Mednih Rud i Koncentratov; Metallurgy: Moscow, Russia, 1966. [Google Scholar]
- Mayangsari, W.; Prasetyo, A.B. Phase Transformation of Limonite Nickel Ores with Na2SO4 Addition in Selective Reduction Process. IOP Conf. Ser. Mater. Sci. Eng. 2017, 202, 012016. [Google Scholar] [CrossRef]
- Dunn, J.G.; Howes, V.L. The oxidation of violarite. Thermochim. Acta 1996, 282–283, 305–316. [Google Scholar] [CrossRef]
- Živković, Ž. Theory of Metallurgical Processes/Teorija Mealurških Proces; University of Belgrade, Technical Faculty in Bor: Belgrade, Serbia, 1991. [Google Scholar]







| Element | Fe | S | Ni | Si | Cu | Ca | Mn | Pb |
|---|---|---|---|---|---|---|---|---|
| Mass % | 41.63 | 25.90 | 5.12 | 4.8 | 0.4 | 2.74 | 0.034 | 0.016 |
| Temperature K | Spectrum | Species |
|---|---|---|
| 298 | 1 | quartz (SiO2) |
| 2 | pentlandite ((Fe,Ni)9S8) | |
| 3 | pyrite (FeS2) | |
| 773 | 4 | NiS + hematite (Fe2O3) |
| 5 | pentlandite ((Fe,Ni)9S8) + quartz (SiO2) | |
| 6 | pentlandite ((Fe,Ni)9S8) + gangue minerals | |
| 873 | 7 | NiS + hematite (Fe2O3) |
| 8 | NiS + wustite (FeO) + quartz (SiO2) | |
| 9 | trevorite (NiFe2O4) | |
| 1073 | 10 | NiS + hematite (Fe2O3) + gangue minerals |
| 11 | trevorite (NiFe2O4) + hematite (Fe2O3) | |
| 12 | magnetite (Fe3O4) + trevorite (NiFe2O4) |
| System | Theoretical Reaction Path |
|---|---|
| Ni-S-O | 773 K 923 K 1073 K |
| Fe-S-O | 773 K 923 K 1073 K |
| Reaction | |||
|---|---|---|---|
| 773 K | 923 K | 1073 K | |
| −543 | −552 | −562 | |
| −1439 | −1404 | −1368 | |
| −270 | −273 | −275 | |
| −257 | −261 | −269 | |
| −912 | −870 | −824 | |
| −231 | −148 | −66 | |
| −2254 | −2232 | −2210 | |
| −283 | −284 | −283 | |
| −271 | −281 | −291 | |
| −266 | −220 | −175 | |
| −1829 | −1805 | −1786 | |
| −259 | −278 | −299 | |
| −1440 | −1388 | −1337 | |
| −201 | −72 | 55 | |
| −230 | −108 | 14 | |
| −129 | −71 | −13 | |
| Temperature Range (K) | Stage | Ea (kJ Mol−1) |
|---|---|---|
| 667–856 | I | 113 |
| 901–1023 | II | 156 |
| 1089–1223 | III | 346 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Božinović, K.; Štrbac, N.; Mitovski, A.; Sokić, M.; Minić, D.; Marković, B.; Stojanović, J. Thermal Decomposition and Kinetics of Pentlandite-Bearing Ore Oxidation in the Air Atmosphere. Metals 2021, 11, 1364. https://doi.org/10.3390/met11091364
Božinović K, Štrbac N, Mitovski A, Sokić M, Minić D, Marković B, Stojanović J. Thermal Decomposition and Kinetics of Pentlandite-Bearing Ore Oxidation in the Air Atmosphere. Metals. 2021; 11(9):1364. https://doi.org/10.3390/met11091364
Chicago/Turabian StyleBožinović, Kristina, Nada Štrbac, Aleksandra Mitovski, Miroslav Sokić, Duško Minić, Branislav Marković, and Jovica Stojanović. 2021. "Thermal Decomposition and Kinetics of Pentlandite-Bearing Ore Oxidation in the Air Atmosphere" Metals 11, no. 9: 1364. https://doi.org/10.3390/met11091364
APA StyleBožinović, K., Štrbac, N., Mitovski, A., Sokić, M., Minić, D., Marković, B., & Stojanović, J. (2021). Thermal Decomposition and Kinetics of Pentlandite-Bearing Ore Oxidation in the Air Atmosphere. Metals, 11(9), 1364. https://doi.org/10.3390/met11091364