Galvanic Effect and Alternating Current Corrosion of Steel in Acidic Red Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Simulated Solution Preparation
2.2. Electrochemical Measurements
2.3. Weight Loss Tests
2.4. Corrosion Products Characterization
2.5. Statistical Method
3. Results and Discussion
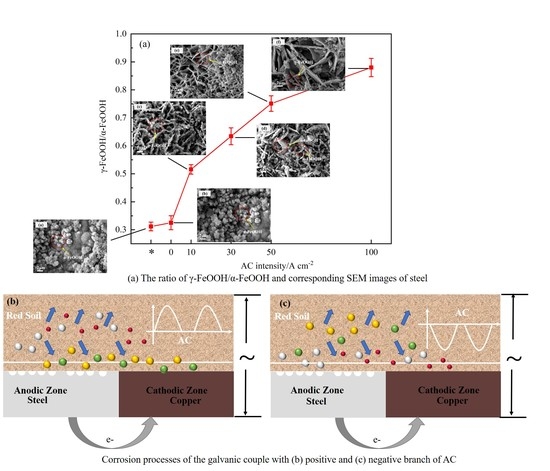
3.1. Weight Loss and Corrosion Product Analyses
3.2. Electrochemical Tests
3.3. AC Corrosion Model for the Galvanic Couple
3.4. Variance Analysis Based on ANOVA
3.5. Corrosion Mechanism of Coupled Steel–Copper Electrodes with AC Interference
4. Conclusions
- (1).
- Based on the results of electrochemical and mass loss experiments, the corrosion rate of the steel in the steel–copper couple is increased by increasing the AC intensity in a relatively monotonic manner, reaching the maximum value when the applied AC intensity is 100 A/m².
- (2).
- The existence of AC changes the ion migration of the galvanic couple, which inhibits the transformation of γ-FeOOH to α-FeOOH and promotes the growth of γ-FeOOH.
- (3).
- The galvanic effect and AC interference cause a synergistic effect on the corrosion of steel in the steel–copper couple. The AC corrosion of steel is further deteriorated when coupled with copper.
- (4).
- Through the ANOVA via the double-factor, it reveals that the galvanic effect is much more significant than the AC aspect in carbon steel corrosion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Zhang, D. Study of corrosion behavior of copper in 3.5 wt.% NaCl solution containing extracellular polymeric substances of an aerotolerant sulphate-reducing bacteria. Corros. Sci. 2018, 136, 275–284. [Google Scholar] [CrossRef]
- Maruschak, P.; Dzyura, V.; Prentkovskis, O.; Lytvynenko, I.; Polutrenko, M. Microdefects of Biocorroded Pipe Steel Surfaces and Safety Assessment of Localized Stress Concentrators. Metals 2020, 10, 852. [Google Scholar] [CrossRef]
- Marušić, K.; Kekez, K.; Martinez, S. Comparison of soil properties measurements in pipeline corrosion estimation. Mater. Corros. 2019, 70, 1700–1707. [Google Scholar] [CrossRef]
- Wasim, M.; Shoaib, S.; Mubarak, N.M.; Inamuddin; Asiri, A.M. Factors influencing corrosion of metal pipes in soils. Environ. Chem. Lett. 2018, 16, 861–879. [Google Scholar] [CrossRef]
- Wang, S.; Yin, X.; Zhang, H.; Liu, D.; Du, N. Coupling effects of pH and dissolved oxygen on the corrosion behavior and mechanism of X80 steel in acidic soil simulated solution. Materials 2019, 12, 3175. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.; Wang, Z.; Wang, D.; Yin, S. A synergistic effect of dissolved oxygen, HCO3-, and Cl− on the electrochemical corrosion behavior of X70 pipeline steel in the oilfield soil environment. Appl. Phys. A 2020, 126, 868. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Wang, Y.; Xu, S.; Yang, X. Chloride-induced stray current corrosion of Q235A steel and prediction model. Constr. Build. Mater. 2019, 219, 164–175. [Google Scholar] [CrossRef]
- Lindström, R.; Svensson, J.-E.; Johansson, L.G. The Influence of Salt Deposits on the Atmospheric Corrosion of Zinc. The Important Role of the Sodium Ion. J. Electrochem. Soc. 2002, 149, B57. [Google Scholar] [CrossRef]
- Shekhar, A.; Kontos, E.; Ramírez-Elizondo, L.; Rodrigo-Mor, A.; Bauer, P. Grid capacity and efficiency enhancement by operating medium voltage AC cables as DC links with modular multilevel converters. Int. J. Electr. Power 2017, 93, 479–493. [Google Scholar] [CrossRef]
- Jones, D. Effect of alternating current on corrosion of low alloy and carbon steels. Corrosion 1978, 34, 428–433. [Google Scholar] [CrossRef]
- Chen, Z.; Qin, C.; Tang, J.; Zhou, Y. Experiment research of dynamic stray current interference on buried gas pipeline from urban rail transit. J. Nat. Gas Sci. Eng. 2013, 15, 76–81. [Google Scholar] [CrossRef]
- Guo, Y.-B.; Liu, C.; Wang, D.-G.; Liu, S.-H. Effects of alternating current interference on corrosion of X60 pipeline steel. Pet. Sci. 2015, 12, 316–324. [Google Scholar] [CrossRef] [Green Version]
- Bertolini, L.; Carsana, M.; Pedeferri, P. Corrosion behaviour of steel in concrete in the presence of stray current. Corros. Sci. 2007, 49, 1056–1068. [Google Scholar] [CrossRef]
- Goidanich, S.; Lazzari, L.; Ormellese, M. AC corrosion—Part 1: Effects on overpotentials of anodic and cathodic processes. Corros. Sci. 2010, 52, 491–497. [Google Scholar] [CrossRef]
- Goidanich, S.; Lazzari, L.; Ormellese, M. AC corrosion. Part 2: Parameters influencing corrosion rate. Corros. Sci. 2010, 52, 916–922. [Google Scholar] [CrossRef]
- Kuang, D.; Cheng, Y.F. Understand the AC induced pitting corrosion on pipelines in both high pH and neutral pH carbonate/bicarbonate solutions. Corros. Sci. 2014, 85, 304–310. [Google Scholar] [CrossRef]
- Qiao, C.; Shen, L.; Hao, L.; Mu, X.; Dong, J.; Ke, W.; Liu, J.; Liu, B. Corrosion kinetics and patina evolution of galvanized steel in a simulated coastal-industrial atmosphere. J. Mater. Sci. Technol. 2019, 35, 2345–2356. [Google Scholar] [CrossRef]
- Wei, J.; Dong, J.; Zhou, Y.; He, X.; Wang, C.; Ke, W. Influence of the secondary phase on micro galvanic corrosion of low carbon bainitic steel in NaCl solution. Mater. Charact. 2018, 139, 401–410. [Google Scholar] [CrossRef]
- Anaman, S.Y.; Cho, H.-H.; Das, H.; Baik, S.-I.; Hong, S.-T.; Lee, J.-S. Galvanic corrosion assessment of friction stir butt welded joint of aluminum and steel alloys. Int. J. Precis. Eng. Manuf. Green Technol. 2020, 7, 905–911. [Google Scholar] [CrossRef]
- Mai, W.; Soghrati, S. New phase field model for simulating galvanic and pitting corrosion processes. Electrochim. Acta 2018, 260, 290–304. [Google Scholar] [CrossRef]
- Snihirova, D.; Höche, D.; Lamaka, S.; Mir, Z.; Hack, T.; Zheludkevich, M.L. Galvanic corrosion of Ti6Al4V-AA2024 joints in aircraft environment: Modelling and experimental validation. Corros. Sci. 2019, 157, 70–78. [Google Scholar] [CrossRef]
- Shan, M.; Guo, K.; Gou, G.; Fu, Z.; Yang, B.; Lu, W. Effect of anodizing on galvanic corrosion behavior of T300 CFRP/5083P-O Al bolted joints. Mater. Corros. 2020, 71, 409–418. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, J.; Cheng, X.; Huang, Y.; Lu, L.; Li, X. Galvanic corrosion of the anodized 7050 aluminum alloy coupled with the low hydrogen embrittlement CdTi plated 300M steel in an industrial-marine atmospheric environment. Surf. Coat. Technol. 2020, 382, 125171. [Google Scholar] [CrossRef]
- Shi, L.; Yang, X.; Song, Y.; Liu, D.; Dong, K.; Shan, D.; Han, E.-H. Effect of corrosive media on galvanic corrosion of complicated tri-metallic couples of 2024 Al alloy/Q235 mild steel/304 stainless steel. J. Mater. Sci. Technol. 2019, 35, 1886–1893. [Google Scholar] [CrossRef]
- Kolesnikov, A.; Kolek, M.; Dohmann, J.F.; Horsthemke, F.; Börner, M.; Bieker, P.; Winter, M.; Stan, M.C. Galvanic corrosion of lithium-powder-based electrodes. Adv. Energy Mater. 2020, 10, 2000017. [Google Scholar] [CrossRef] [Green Version]
- Ni, Q.; Xia, X.; Zhang, J.; Dai, N.; Fan, Y. Electrochemical and SVET Studies on the Typical Polarity Reversal of Cu–304 Stainless Steel Galvanic Couple in Cl−-Containing Solution with Different pH. Electrochim. Acta 2017, 247, 207–215. [Google Scholar] [CrossRef]
- Ikeuba, A.I.; Zhang, B.; Wang, J.; Han, E.-H.; Ke, W. Understanding the galvanic corrosion of the Q-phase/Al couple using SVET and SIET. J. Mater. Sci. Technol. 2019, 35, 1444–1454. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 8407:2021. Corrosion of Metals and Alloys—Removal of Corrosions Products from Corrosion Test Specimens; International Organization for Standardization: Geneva, Switzerland, 2021. [Google Scholar]
- Elmushyakhi, A. Parametric characterization of nano-hybrid wood polymer composites using ANOVA and regression analysis. Structures 2021, 29, 652–662. [Google Scholar] [CrossRef]
- Li, J.; Su, H.; Chai, F.; Chen, X.-p.; Li, X.-y.; Meng, H.-m. Simulated corrosion test of Q235 steel in diatomite soil. J. Iron Steel Res. Int. 2015, 22, 352–360. [Google Scholar] [CrossRef]
- Tang, D.; Du, Y.; Lu, M.; Jiang, Z.; Dong, L.; Wang, J. Effect of AC current on corrosion behavior of cathodically protected Q235 steel. Mater. Corros. 2015, 66, 278–285. [Google Scholar] [CrossRef]
- He, B.; Han, P.; Hou, L.; Zhang, D.; Bai, X. Understanding the effect of soil particle size on corrosion behavior of natural gas pipeline via modelling and corrosion micromorphology. Eng. Fail. Anal. 2017, 80, 325–340. [Google Scholar] [CrossRef]
- Dai, N.; Zhang, J.; Chen, Q.; Yi, B.; Cao, F.; Zhang, J. Effect of the direct current electric field on the initial corrosion of steel in simulated industrial atmospheric environment. Corros. Sci. 2015, 99, 295–303. [Google Scholar] [CrossRef]
- Shao, Y.; Mu, M.; Zhang, B.; Nie, K.; Liao, Q. Corrosion Behavior of Copper-Clad Steel Bars with Unclad Two-End Faces for Grounding Grids in the Red Clay Soil. J. Mater. Eng. Perform. 2017, 26, 1751–1757. [Google Scholar] [CrossRef]
- Shi, J.; Ming, J.; Zhang, Y.; Jiang, J. Corrosion products and corrosion-induced cracks of low-alloy steel and low-carbon steel in concrete. Cem. Concr. Compos. 2018, 88, 121–129. [Google Scholar] [CrossRef]
- Wang, G.; Wu, Q.; Li, X.-Z.; Xu, J.; Xu, Y.; Shi, W.-H.; Wang, S.-L. Microscopic analysis of steel corrosion products in seawater and sea-sand concrete. Materials 2019, 12, 3330. [Google Scholar] [CrossRef] [Green Version]
- Pang, L.; Wang, Z.; Zheng, Y.; Lai, X.; Han, X. On the localised corrosion of carbon steel induced by the in-situ local damage of porous corrosion products. J. Mater. Sci. Technol. 2020, 54, 95–104. [Google Scholar] [CrossRef]
- Dai, N.; Chen, Q.; Zhang, J.; Zhang, X.; Ni, Q.; Jiang, Y.; Li, J. The corrosion behavior of steel exposed to a DC electric field in the simulated wet-dry cyclic environment. Mater. Chem. Phys. 2017, 192, 190–197. [Google Scholar] [CrossRef]
- Singh, J.K.; Singh, D.D.N. The nature of rusts and corrosion characteristics of low alloy and plain carbon steels in three kinds of concrete pore solution with salinity and different pH. Corros. Sci. 2012, 56, 129–142. [Google Scholar] [CrossRef]
- Fan, X.-L.; Chen, Y.-X.; Zhang, J.-X.; Lin, D.-Y.; Liu, X.-X.; Xia, X.-J. Galvanic Corrosion Behavior of Copper–Drawn Steel for Grounding Grids in the Acidic Red Soil Simulated Solution. Acta Metall. Sin. 2020, 33, 1571–1582. [Google Scholar] [CrossRef]
- Schwertmann, U.; Murad, E. Effect of pH on the formation of goethite and hematite from ferrihydrite. Clays Clay Miner. 1983, 31, 277–284. [Google Scholar] [CrossRef]
- De la Fuente, D.; Díaz, I.; Simancas, J.; Chico, B.; Morcillo, M. Long-term atmospheric corrosion of mild steel. Corros. Sci. 2011, 53, 604–617. [Google Scholar] [CrossRef] [Green Version]
- Antunes, R.A.; Costa, I.; de Faria, D.L.A. Characterization of corrosion products formed on steels in the first months of atmospheric exposure. Mater. Res. 2003, 6, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Monnier, J.; Réguer, S.; Foy, E.; Testemale, D.; Mirambet, F.; Saheb, M.; Dillmann, P.; Guillot, I. XAS and XRD in situ characterisation of reduction and reoxidation processes of iron corrosion products involved in atmospheric corrosion. Corros. Sci. 2014, 78, 293–303. [Google Scholar] [CrossRef]
- Jia, J.; Cheng, X.; Yang, X.; Li, X.; Li, W. A study for corrosion behavior of a new-type weathering steel used in harsh marine environment. Constr. Build. Mater. 2020, 259, 119760. [Google Scholar] [CrossRef]
- Wei, B.; Qin, Q.; Bai, Y.; Yu, C.; Xu, J.; Sun, C.; Ke, W. Short-period corrosion of X80 pipeline steel induced by AC current in acidic red soil. Eng. Fail. Anal. 2019, 105, 156–175. [Google Scholar] [CrossRef]
- Bosch, R.-W.; Bogaerts, W. A theoretical study of AC-induced corrosion considering diffusion phenomena. Corros. Sci. 1998, 40, 323–336. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Z.; Yang, C.; Gou, J. The AC Corrosion Mechanisms and Models: A Review. Corrosion 2020, 76, 188–201. [Google Scholar] [CrossRef]
- Sidhom, H.; Amadou, T.; Braham, C. Evaluation by the Double Loop Electrochemical Potentiokinetic Reactivation Test of Aged Ferritic Stainless Steel Intergranular Corrosion Susceptibility. Metall. Mater. Trans. A 2010, 41, 3136–3150. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zeng, H.; Wu, Z.; Cao, J. Impact of sodium hypochlorite cleaning on the surface properties and performance of PVDF membranes. Appl. Surf. Sci. 2018, 428, 289–295. [Google Scholar] [CrossRef]
- Ishikawa, T.; Takeuchi, K.; Kandori, K.; Nakayama, T. Transformation of γ-FeOOH to α-FeOOH in acidic solutions containing metal ions. Colloids Surf. A 2005, 266, 155–159. [Google Scholar] [CrossRef]
- Gu, J.; Xiao, Y.; Dai, N.; Zhang, X.; Ni, Q.; Zhang, J. The Suppression of transformation of γ-FeOOH to α-FeOOH accelerating the steel corrosion in simulated industrial atmospheric environment with a DC electric field interference. Corros. Eng. Sci. Technol. 2019, 54, 249–256. [Google Scholar] [CrossRef]
- Hashimoto, K.; Misawa, T. The solubility of γ-FeOOH in perchloric acid at 25 °C. Corros. Sci. 1973, 13, 229–231. [Google Scholar] [CrossRef]
- Misawa, T. The thermodynamic consideration for Fe-H2O system at 25 °C. Corros. Sci. 1973, 13, 659–676. [Google Scholar] [CrossRef]














| C | Mn | Si | S | Fe |
|---|---|---|---|---|
| 0.21 | 0.46 | 0.24 | 0.03 | Bal. |
| Cl− | SO4− | HCO3− | Ca2+ | Na+ |
|---|---|---|---|---|
| 0.0084 | 0.0054 | 0.001 | 0.0023 | 0.0032 |
| NaCl | CaCl2 | Na2SO4 | NaHCO3 |
|---|---|---|---|
| 0.038 | 0.023 | 0.054 | 0.01 |
| i/A·m−² | 0 | 10 | 30 | 50 | 100 |
|---|---|---|---|---|---|
| Eg/mV vs SCE | −659.7 | −573.53 | −576.16 | −577.36 | −607.72 |
| ig/A·cm−² | 9.0741 × 10–5 | 1.3776 × 10–4 | 1.7513 × 10–4 | 2.0844 × 10–4 | 2.2405 × 10–4 |
| Source | Three Types of Sums of Squares | Degree of Freedom | Mean Square | F | Significance |
|---|---|---|---|---|---|
| Revised model | 5947.060α | 9 | 660.784 | 56.751 | 0.000 |
| Intercept | 134,526.792 | 1 | 134,526.792 | 11,553.836 | 0.000 |
| Galvanic effect | 2535.172 | 1 | 2535.172 | 217.733 | 0.000 |
| AC | 2920.531 | 4 | 730.133 | 62.707 | 0.000 |
| Error | 232.869 | 20 | 11.643 | \ | \ |
| Total | 140,706.722 | 30 | \ | \ | \ |
| Revised total | 6179.929 | 29 | \ | \ | \ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.-W.; Zhang, J.-X.; Gao, Y.; Dai, N.-W.; Chen, Y.-X.; Lin, D.-Y.; Xia, X.-J. Galvanic Effect and Alternating Current Corrosion of Steel in Acidic Red Soil. Metals 2022, 12, 296. https://doi.org/10.3390/met12020296
Wang Q-W, Zhang J-X, Gao Y, Dai N-W, Chen Y-X, Lin D-Y, Xia X-J. Galvanic Effect and Alternating Current Corrosion of Steel in Acidic Red Soil. Metals. 2022; 12(2):296. https://doi.org/10.3390/met12020296
Chicago/Turabian StyleWang, Qi-Wei, Jun-Xi Zhang, Yan Gao, Nian-Wei Dai, Yun-Xiang Chen, De-Yuan Lin, and Xiao-Jian Xia. 2022. "Galvanic Effect and Alternating Current Corrosion of Steel in Acidic Red Soil" Metals 12, no. 2: 296. https://doi.org/10.3390/met12020296
APA StyleWang, Q. -W., Zhang, J. -X., Gao, Y., Dai, N. -W., Chen, Y. -X., Lin, D. -Y., & Xia, X. -J. (2022). Galvanic Effect and Alternating Current Corrosion of Steel in Acidic Red Soil. Metals, 12(2), 296. https://doi.org/10.3390/met12020296