Elemental Fluctuation in Gd3Al2Ga3O12:Ce Crystals Imposed by Li+ and Mg2+ Co-Doping: The Impact on Defects, Luminescence, and Scintillation Properties
Abstract
:1. Introduction
2. Methodology
2.1. Crystal Growth
2.2. Powder X-ray Diffraction and Energy-Dispersive X-ray Spectroscopy Analysis
2.3. Optical, Luminescence, and Scintillation Characteristics
2.4. Thermoluminescence Properties
3. Results and Discussion
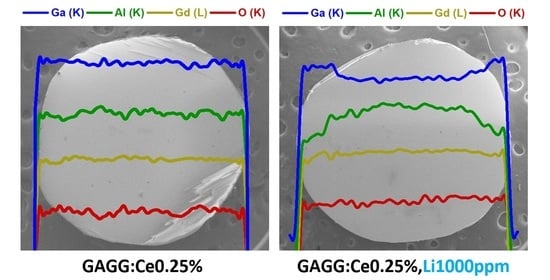
3.1. Impact of Li+ and Mg2+ on Atom Distribution and Crystal Phase by XRD and SEM-EDS Analysis
3.2. Optical and Photoluminescence Characterization under Synchrotron Radiation
3.3. Thermally Stimulated Luminescence Characteristics
3.4. Scintillation Characteristics
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, Z.; Liu, Q. Progress in Discovery and Structural Design of Color Conversion Phosphors for LEDs. Prog. Mater. Sci. 2016, 84, 59–117. [Google Scholar] [CrossRef]
- Massey, G.A.; Yarborough, J.M. High Average Power Operation and Nonlinear Optical Generation with the Nd:YALO3laser. Appl. Phys. Lett. 1971, 18, 576–579. [Google Scholar] [CrossRef]
- Struve, B.; Huber, G.; Laptev, V.V.; Shcherbakov, I.A.; Zharikov, E.V. Tunable Room-Temperature Cw Laser Action in Cr3+: GdScGa-Garnet. Appl. Phys. B 1983, 30, 117–120. [Google Scholar] [CrossRef]
- Nikl, M.; Yoshikawa, A. Recent R&D Trends in Inorganic Single-Crystal Scintillator Materials for Radiation Detection. Adv. Opt. Mater. 2015, 3, 463–481. [Google Scholar] [CrossRef]
- Kanke, Y.; Navrotsky, A. A Calorimetric Study of the Lanthanide Aluminum Oxides and the Lanthanide Gallium Oxides: Stability of the Perovskites and the Garnets. J. Solid State Chem. 1998, 141, 424–436. [Google Scholar] [CrossRef]
- Xia, Z.; Meijerink, A. Ce3+-Doped Garnet Phosphors: Composition Modification, Luminescence Properties and Applications. Chem. Soc. Rev. 2017, 46, 275–299. [Google Scholar] [CrossRef] [Green Version]
- Bartosiewicz, K.; Babin, V.; Beitlerova, A.; Bohacek, P.; Jurek, K.; Nikl, M. The Temperature Dependence Studies of Rare-Earth (Dy3+, Sm3+, Eu3+ and Tb3+) Activated Gd3Ga3Al2O12 Garnet Single Crystals. J. Lumin. 2017, 189, 126–139. [Google Scholar] [CrossRef]
- Ueda, J.; Miyano, S.; Tanabe, S. Formation of Deep Electron Traps by Yb3+ Codoping Leads to Super-Long Persistent Luminescence in Ce3+-Doped Yttrium Aluminum Gallium Garnet Phosphors. ACS Appl. Mater. Interfaces 2018, 10, 20652–20660. [Google Scholar] [CrossRef]
- Kamada, K.; Yanagida, T.; Endo, T.; Tsutumi, K.; Usuki, Y.; Nikl, M.; Fujimoto, Y.; Fukabori, A.; Yoshikawa, A. 2inch Diameter Single Crystal Growth and Scintillation Properties of Ce:Gd3Al2Ga3O12. J. Cryst. Growth 2012, 352, 88–90. [Google Scholar] [CrossRef]
- Sibczynski, P.; Iwanowska-Hanke, J.; Moszyński, M.; Swiderski, L.; Szawłowski, M.; Grodzicka, M.; Szczęśniak, T.; Kamada, K.; Yoshikawa, A. Characterization of GAGG:Ce Scintillators with Various Al-to-Ga Ratio. Nucl. Instrum. Methods Phys. Res. A 2015, 772, 112–117. [Google Scholar] [CrossRef]
- Lucchini, M.T.; Babin, V.; Bohacek, P.; Gundacker, S.; Kamada, K.; Nikl, M.; Petrosyan, A.; Yoshikawa, A.; Auffray, E. Effect of Mg2+ Ions Co-Doping on Timing Performance and Radiation Tolerance of Cerium Doped Gd3Al2Ga3O12 Crystals. Nucl. Instrum. Methods Phys. Res. A 2016, 816, 176–183. [Google Scholar] [CrossRef]
- Auffray, E.; Augulis, R.; Fedorov, A.; Dosovitskiy, G.; Grigorjeva, L.; Gulbinas, V.; Koschan, M.; Lucchini, M.; Melcher, C.; Nargelas, S.; et al. Excitation Transfer Engineering in Ce-Doped Oxide Crystalline Scintillators by Codoping with Alkali-Earth Ions. Phys. Status Solidi 2018, 215, 1700798. [Google Scholar] [CrossRef]
- Lucchini, M.T.; Gundacker, S.; Lecoq, P.; Benaglia, A.; Nikl, M.; Kamada, K.; Yoshikawa, A.; Auffray, E. Timing Capabilities of Garnet Crystals for Detection of High Energy Charged Particles. Nucl. Instrum. Methods Phys. Res. A 2017, 852, 1–9. [Google Scholar] [CrossRef]
- Zazubovich, S.; Laguta, V.V.; Machek, P.; Kamada, K.; Yoshikawa, A.; Nikl, M. Effect of Li+ Co-Doping on the Luminescence and Defects Creation Processes in Gd3(Ga,Al)5O12:Ce Scintillation Crystals. J. Lumin. 2022, 242, 118548. [Google Scholar] [CrossRef]
- Laguta, V.; Buryi, M.; Babin, V.; Machek, P.; Zazubovich, S.; Bartosiewicz, K.; Kurosawa, S.; Yamaji, A.; Yoshikawa, A.; Uličná, K.; et al. Li+ Incorporation and Defect-Creation Processes Imposed by X-Ray and UV Irradiation in Li-Codoped Y3Al5O12:Ce Scintillation Crystals. J. Mater. Chem. C Mater. Opt. Electron. Devices 2023, 11, 1346. [Google Scholar] [CrossRef]
- Yoshino, M.; Bartosiewicz, K.; Horiai, T.; Kamada, K.; Yamaji, A.; Shoji, Y.; Yokota, Y.; Kurosawa, S.; Ohashi, Y.; Sato, H.; et al. Relationship between Li/Ce Concentration and the Luminescence Properties of Codoped Gd3 (Ga, Al)5O12:Ce. Phys. Status Solidi B Basic Res. 2020, 257, 1900504. [Google Scholar] [CrossRef]
- Bartosiewicz, K.; Markovskyi, A.; Horiai, T.; Szymański, D.; Kurosawa, S.; Yamaji, A.; Yoshikawa, A.; Zorenko, Y. A Study of Mg2+ Ions Effect on Atoms Segregation, Defects Formation, Luminescence and Scintillation Properties in Ce3+ Doped Gd3Al2Ga3O12 Single Crystals. J. Alloys Compd. 2022, 905, 164154. [Google Scholar] [CrossRef]
- Bartosiewicz, K.; Babin, V.; Kamada, K.; Yoshikawa, A.; Kurosawa, S.; Beitlerova, A.; Kucerkova, R.; Nikl, M.; Zorenko, Y. Ga for Al Substitution Effects on the Garnet Phase Stability and Luminescence Properties of Gd3GaxAl5-XO12:Ce Single Crystals. J. Lumin. 2019, 216, 116724. [Google Scholar] [CrossRef]
- Babin, V.; Herman, P.; Kucera, M.; Nikl, M.; Zazubovich, S. Effect of Mg2+ Co-Doping on the Photo- and Thermally Stimulated Luminescence of the (Lu,Gd)3(Ga,Al)5O12:Ce Epitaxial Films. J. Lumin. 2019, 215, 116608. [Google Scholar] [CrossRef]
- Song, Z.; Zhou, D.; Liu, Q. Tolerance Factor and Phase Stability of the Garnet Structure. Acta Crystallogr. C Struct. Chem. 2019, 75 Pt 10, 1353–1358. [Google Scholar] [CrossRef]
- Sickafus, K.E.; Melcher, C.L.; Flynn-Hepford, M.I.; Wang, Y.; Jaroslaw, G.; Smith, J.P.; Drewry, S.M.; Zhuravleva, M. Crystal Chemistry of Rare-Earth Containing Garnets: Prospects for High Configurational Entropy. J. Solid State Chem. 2022, 310, 122997. [Google Scholar] [CrossRef]
- Wong, C.M.; Rotman, S.R.; Warde, C. Optical Studies of Cerium Doped Yttrium Aluminum Garnet Single Crystals. Appl. Phys. Lett. 1984, 44, 1038–1040. [Google Scholar] [CrossRef]
- Tanner, P.A.; Fu, L.; Ning, L.; Cheng, B.-M.; Brik, M.G. Soft Synthesis and Vacuum Ultraviolet Spectra of YAG:Ce3+ nanocrystals: Reassignment of Ce3+ energy Levels. J. Phys. Condens. Matter 2007, 19, 216213. [Google Scholar] [CrossRef]
- Bartosiewicz, K.; Babin, V.; Kamada, K.; Yoshikawa, A.; Nikl, M. Energy Migration Processes in Undoped and Ce-Doped Multicomponent Garnet Single Crystal Scintillators. J. Lumin. 2015, 166, 117–122. [Google Scholar] [CrossRef]
- Khanin, V.; Venevtsev, I.; Chernenko, K.; Pankratov, V.; Klementiev, K.; van Swieten, T.; van Bunningen, A.J.; Vrubel, I.; Shendrik, R.; Ronda, C.; et al. Exciton Interaction with Ce3+ and Ce4+ Ions in (LuGd)3(Ga,Al)5O12 Ceramics. J. Lumin. 2021, 237, 118150. [Google Scholar] [CrossRef]
- Babin, V.; Bohacek, P.; Grigorjeva, L.; Kučera, M.; Nikl, M.; Zazubovich, S.; Zolotarjovs, A. Effect of Mg2+ Ions Co-Doping on Luminescence and Defects Formation Processes in Gd3(Ga,Al)5O12:Ce Single Crystals. Opt. Mater. 2017, 66, 48–58. [Google Scholar] [CrossRef]









| Crystal | Al at.% (Kα X-ray)/Number of Atoms | Ga at.% (Kα X-ray)/Number of Atoms | Gd at.% (Lα X-ray)/Number of Atoms | |||
|---|---|---|---|---|---|---|
| Rim | Core | Rim | Core | Rim | Core | |
| GAGG:Ce3+ 0.25% | 25.89/2.07 | 26.21/2.10 | 37.13/2.97 | 36.89/2.95 | 36.98/2.96 | 36.90/2.95 |
| GAGG:Ce3+ 0.25%, Li+ 1000 ppm | 24.93/1.99 | 28.05/2.24 | 38.07/3.05 | 35.03/2.80 | 37.00/2.96 | 36.92/2.95 |
| GAGG:Ce3+ 0.25%, Mg2+ 1000 ppm | 23.25/1.86 | 28.83/2.41 | 39.86/3.19 | 34.11/2.73 | 36.89/2.95 | 37.06/2.96 |
| Sample | Scintillation Decay Constants | Light Yield (Phot/1 MeV) [2 μs] | |
|---|---|---|---|
| τ1 [ns]/% | τ2 [ns]/% | ||
| GAGG:Ce3+ 0.25% | 50/61 | 225/39 | 31,500 ± 1100 |
| GAGG:Ce3+ 0.25%, Li+ 1000 ppm | 58/89 | 240/11 | 33,200 ± 1400 |
| GAGG:Ce3+ 0.25%, Mg2+ 1000 ppm | 19/42 | 80/58 | 21,500 ± 800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartosiewicz, K. Elemental Fluctuation in Gd3Al2Ga3O12:Ce Crystals Imposed by Li+ and Mg2+ Co-Doping: The Impact on Defects, Luminescence, and Scintillation Properties. Metals 2023, 13, 422. https://doi.org/10.3390/met13020422
Bartosiewicz K. Elemental Fluctuation in Gd3Al2Ga3O12:Ce Crystals Imposed by Li+ and Mg2+ Co-Doping: The Impact on Defects, Luminescence, and Scintillation Properties. Metals. 2023; 13(2):422. https://doi.org/10.3390/met13020422
Chicago/Turabian StyleBartosiewicz, Karol. 2023. "Elemental Fluctuation in Gd3Al2Ga3O12:Ce Crystals Imposed by Li+ and Mg2+ Co-Doping: The Impact on Defects, Luminescence, and Scintillation Properties" Metals 13, no. 2: 422. https://doi.org/10.3390/met13020422
APA StyleBartosiewicz, K. (2023). Elemental Fluctuation in Gd3Al2Ga3O12:Ce Crystals Imposed by Li+ and Mg2+ Co-Doping: The Impact on Defects, Luminescence, and Scintillation Properties. Metals, 13(2), 422. https://doi.org/10.3390/met13020422