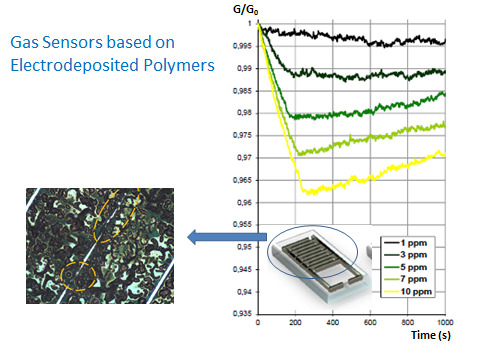
Gas Sensors Based on Electrodeposited Polymers
Abstract
:1. Introduction
2. The Different Configurations of Polymer-Based Gas Sensors

3. Gas Sensors Based on Conducting Electrodeposited Polymers and Their Derivatives
3.1. Gas Sensors Based on Electrodeposited Polymer Films
| Polymer | Dopant (D) or Side Chain (S) | Analyte | Reference |
|---|---|---|---|
| Polypyrrole | D: tetrafluoroborate | Carbon dioxide | [27] |
| Polypyrrole | D: decane sulfonate, butane sulfonate, methylphosphonic acid | Ethanol | [19] |
| Polypyrrole | D: 12 different dopants mostly including sulfonated anions | Benzene, toluene, ethyl benzene, xylene | [29] |
| Polypyrrole | D: 7 different dopants with perchlorate, phosphate and sulfonate anions | Ethanol, methanol, acetone, butanone, pentanone | [30] |
| Polypyrrole | D: perchlorate | Ammonia | [22] |
| Polypyrrole | D: 5 dopants perchlorate, phosphate, borate and sulfonate anions | Ammonia | [23] |
| Polypyrrole | D: perchlorate | Ammonia | [24] |
| Polypyrrole | D: lithium perchlorate and toluenesulfonic acid | Ammonia | [32] |
| Polypyrrole | D: sulfonated polyaniline | Ammonia | [33] |
| Polypyrrole (nanowires) | D: sodium dodecyl sulfate | Ammonia | [34] |
| Polyaniline | D: sulfuric acid | Ethanol, acetone | [20] |
| Polyaniline | D: Keggin type 12-tungstophosphoric acid | Acetone | [28] |
| Polyaniline (nanowires) | D: sulfuric acid | Ammonia | [35] |
| Polypyrrole derivative | S: hydroxyalkyl and carboxyalkyl | Alcohols, hexane, triethylamine, toluene, acetonitrile | [36,37] |
| Polypyrrole derivative | S: alkoxy | HCl, ammonia, NO, oxygen | [38] |
| Polypyrrole derivative | S: alkyl, sulfonic acid, ammonium, esters alkoxy | Ethanol, acetonitrile | [39] |
| PEDOT derivative | S: ether | Ethanol, acetonitrile | [40] |

3.2. Gas Sensors Based on Conducting Polymer Nanowires
3.3. Gas Sensors Based on Electrodeposited Polymer Derivative Films
4. Gas Sensors Based on Hybrid Materials
4.1. Polymer/Metallophthalocyanine Hybrid Materials
4.2. Polymer/Metal Nanoparticle Hybrid Materials
| Polymer | Other Element | Analyte | Reference |
|---|---|---|---|
| Polypyrrole | Copper phthalocyanine | Dimethyl methyl phosphonate | [44] |
| Polypyrrole | Sulfonated metallophthalocyanines | NO2 | [45] |
| Polypyrrole | Cobalt phthalocyanine | NH3 | [46,47] |
| Polypyrrole | Pd, Cu | NH3, H2 and CO | [21] |
| Polyaniline | Au | H2S | [48] |
| Polyaniline | SWNT-COOH | NH3 | [40] |
5. Response Mechanism
| Response Mechanism | Gas or Catalyst | Response | Reference |
|---|---|---|---|
| Electron transfer | Electron acceptor (NO2) | Conductivity increase | [31,32,33,3435,45] |
| Electron donor (NH3) | Conductivity decrease | [49,50] | |
| Proton transfer | Acid (HCl, H2S, CO2, CH3COOH) | Conductivity increase | [54,55,56,57,5859] |
| Base (NH3) | Conductivity decrease | [51–53,60,61] | |
| Catalysts (Pd, Cu, C) | DMMP, NH3, H2S | Enhancement of the response (conductivity increase or decrease) | [21,40,48] |
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nylander, C.; Armgarth, M.; Lundstrom, I. An ammonia detector based on a conducting polymer. Proc. Int. Meet. Chem. Sens. 1983, 203–207. [Google Scholar]
- Hu, J.; Wu, X.; Zeng, W. Formaldehyde sensor based on polypyrrole/β-cyclodextrin. J. Control. Release 2011, 152, E211–E213. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez Pineda, E.; Alcaide, F.; Presa, M.J.R.; Bolzan, A.E.; Gervasi, C.A. Electrochemical Preparation and Characterization of Polypyrrole/Stainless Steel Electrodes Decorated with Gold Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 2677–2687. [Google Scholar] [CrossRef] [PubMed]
- Marini, M.; Pilati, F.; Pourabbas, B. Smooth surface polypyrrole-silica core-shell nanoparticles: Preparation, characterization and properties. Macromol. Chem. 2008, 209, 1374–1380. [Google Scholar] [CrossRef]
- Oueiny, C.; Berlioz, S.; Perrin, F.X. Carbon nanotube-polyaniline composites. Prog. Polym. Sci. 2014, 39, 707–748. [Google Scholar] [CrossRef]
- Korostynska, O.; Arshak, K.; Gill, E.; Arshak, A. Review on State-of-the-art in Polymer Based pH Sensors. Sensors 2007, 7, 3027–3042. [Google Scholar] [CrossRef]
- Sulka, G.D.; Hnida, K.; Brzozka, A. pH sensors based on polypyrrole nanowire arrays. Electrochim. Acta 2012, 104, 536–541. [Google Scholar] [CrossRef]
- Braiek, M.; Barhoumi, H.; Maaref, A.; Jaffrezic-Renault, N. Elaboration and Characterization of pH Sensor Based on Polypyrrole Nanowires. Sensors Lett. 2011, 9, 2154–2157. [Google Scholar] [CrossRef]
- Lindfors, T.; Ivaska, A. pH sensitivity of polyaniline and its substituted derivatives. J. Electroanal. Chem. 2002, 531, 43–52. [Google Scholar] [CrossRef]
- Slim, C.; Ktari, N.; Cakara, D.; Kanoufi, F.; Combellas, C. Polyaniline films based ultramicroelectrodes sensitive to pH. J. Electroanal. Chem. 2008, 612, 53–62. [Google Scholar] [CrossRef]
- Cosnier, S.; Holzinger, M. Electrosynthesized polymers for biosensing. Chem. Soc. Rev. 2011, 40, 2146–2156. [Google Scholar] [CrossRef] [PubMed]
- Albanese, D.; Malvano, F.; Sannini, A.; Pilloton, R.; di Matteo, M. A Doped Polyaniline Modified Electrode Amperometric Biosensor for Gluconic Acid Determination in Grapes. Sensors 2014, 14, 11097–11109. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, S.; Yeow, J.T.W. Conductive polymer-based sensor for biomedical applications. Biosens. Bioelectron. 2011, 26, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Bobacka, J.; Ivaska, A. Chemical Sensors Based on Conducting Polymers. In Electropolymerization: Concepts, Materials and Applications; Cosnier, S., Karyakin, A., Eds.; Wiley VCH: Weinheim, Germany, 2010; pp. 173–187. [Google Scholar]
- Cosnier, S.; Holzinger, M. Biosensors Based on Electropolymerized Films. In Electropolymerization: Concepts, Materials and Applications; Cosnier, S., Karyakin, A., Eds.; Wiley VCH: Weinheim, Germany, 2010; pp. 189–213. [Google Scholar]
- Gardon, M.; Guilemany, J.M. A review on fabrication, sensing mechanisms and performance of metal oxide gas sensors. J. Mater. Sci. 2013, 24, 1410–1421. [Google Scholar] [CrossRef]
- Arafat, M.M.; Dinan, B.; Akbar, S.A.; Haseeb, A.S.M.A. Gas Sensors Based on One Dimensional Nanostructured Metal-Oxides: A Review. Sensors 2012, 12, 7207–7258. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Liu, S.B.; Meng, F.L.; Liu, J.Y.; Jin, Z.; Kong, L.T.; Liu, J.H. Metal Oxide Nanostructures and Their Gas Sensing Properties: A Review. Sensors 2012, 12, 2610–2631. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Chetwynd, D.G.; Covington, J.A.; Toh, C.S.; Gardner, J.W. Micro-gas-sensor with conducting polymers. Sens. Actuators B 2002, 84, 66–71. [Google Scholar] [CrossRef]
- Reemts, J.; Parisi, J.; Schlettwein, D. Electrochemical growth of gas-sensitive polyaniline thin films across an insulating gap. Thin Solid Films 2004, 466, 320–325. [Google Scholar] [CrossRef]
- Cho, S.; Kwon, O.S.; You, S.A.; Jang, J. Shape-controlled polyaniline chemiresistors for high-performance DMMP sensors: Effect of morphologies and charge-transport properties. J. Mater. Chem. A 2013, 1, 5679–5688. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, T.; Wu, Z.; Li, D.; Chen, X.; Xie, D. Study on the NH3 gas sensitive properties and sensitive mechanism of polypyrrole. Sens. Actuators B 2000, 66, 280–282. [Google Scholar]
- Patois, T.; Sanchez, J.B.; Berger, F.; Rauch, J.Y.; Fievet, P.; Lakard, B. Ammonia gas sensors based on polypyrrole films: Influence of electrodeposition parameters. Sens. Actuators B 2012, 171–172, 431–439. [Google Scholar] [CrossRef]
- Carquigny, S.; Sanchez, J.B.; Berger, F.; Lakard, B.; Lallemand, F. Ammonia gas sensor based on electrosynthesized polypyrrole films. Talanta 2009, 78, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Albert, K.J.; Lewis, N.S.; Schauer, C.L.; Sotzing, G.A.; Stitzel, S.E.; Vaid, T.P.; Walt, D.R. Cross-reactive chemical sensor arrays. Chem. Rev. 2000, 100, 2595–2626. [Google Scholar] [CrossRef] [PubMed]
- Segut, O.; Lakard, B.; Herlem, G.; Rauch, J.Y.; Jeannot, J.C.; Robert, L.; Fahys, B. Development of miniaturized pH biosensors based on electrosynthesized polymer films. Anal. Chim. Acta 2007, 597, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tebizi-Tighilt, F.Z.; Zane, F.; Belhaneche-Bensemra, N.; Belhousse, S.; Sam, S.; Gabouze, N.E. Electrochemical gas sensors based on polypyrrole-porous silicon. Appl. Surf. Sci. 2013, 269, 180–183. [Google Scholar] [CrossRef]
- Iekha, P.C.; Subramanian, E.; Padiyan, D.P. Electrodeposition of polyaniline thin films doped with dodecatungstophosphoric acid: Effect of annealing and vapor sensing. Sens. Actuators B 2007, 122, 274–281. [Google Scholar]
- Barisci, J.N.; Wallace, G.G.; Andrews, M.K.; Partridge, A.C.; Harris, P.D. Conducting polymer sensors for monitoring aromatic hydrocarbons using an electronic nose. Sens. Actuators B 2002, 84, 252–257. [Google Scholar] [CrossRef]
- Hamilton, S.; Hepher, M.J.; Sommerville, J. Polypyrrole materials for detection and discrimination of volatile organic compounds. Sens. Actuators B 2005, 107, 424–432. [Google Scholar] [CrossRef]
- Timmer, B.; Olthuis, W.; van den Berg, A. Ammonia sensors and their applications—A review. Sens. Actuators B 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Brie, M.; Turcu, R.; Neamtu, C.; Pruneanu, S. The effect of initial conductivity and doping anions on gas sensitivity of conducting polypyrrole films to NH3. Sens. Actuators B 1996, 37, 119–122. [Google Scholar] [CrossRef]
- Bai, H.; Chen, Q.; Li, C.; Lu, C.; Shi, G. Electrosynthesis of polypyrrole/sulfonated polyaniline composite films and their applications for ammonia gas sensing. Polymer 2007, 48, 4015–4020. [Google Scholar] [CrossRef]
- Kim, D.; Yoo, B. A novel electropolymerization method for PPy nanowire-based NH3 gas sensor with low contact resistance. Sens. Actuators B 2011, 160, 1168–1173. [Google Scholar] [CrossRef]
- Tuan, C.V.; Tuan, M.A.; Hieu, N.V.; Trung, T. Electrochemical synthesis of polyaniline nanowires on Pt interdigitated microelectrode for room temperature NH3 gas sensor application. Curr. Appl. Phys. 2012, 12, 1011–1016. [Google Scholar] [CrossRef]
- Al-Mashat, L.; Debiemme-Chouvy, C.; Borensztajn, S.; Wlodarski, W. Electropolymerized Polypyrrole Nanowires for Hydrogen Gas Sensing. J. Phys. Chem. C 2012, 116, 13388–13394. [Google Scholar] [CrossRef]
- Deng, Z.; Stone, D.C.; Thompson, M. Characterization of Polymer Films of Pyrrole Derivatives for Chemical Sensing by Cyclic Voltammetry, X-ray Photoelectron Spectroscopy and Vapour Sorption Studies. Analyst 1997, 122, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Stone, D.C.; Thompson, M. Selective Detection of Aroma Components by Acoustic Wave Sensors Coated with Conducting Polymer Films. Analyst 1996, 121, 671–679. [Google Scholar] [CrossRef]
- Krondak, M.; Broncova, G.; Anikin, S.; Merz, A.; Mirsky, V.M. Chemosensitive properties of poly-4,4′dialkoxy-2,2′bipyrroles. J. Sol. State Electrochem. 2006, 10, 185–191. [Google Scholar] [CrossRef]
- Zhang, T.; Nix, M.B.; Yoo, B.Y.; Deshusses, M.A.; Myung, N.V. Electrochemically functionalized single-walled carbon nanotube gas sensor. Electroanalysis 2006, 18, 1153–1158. [Google Scholar] [CrossRef]
- Vercelli, B.; Zecchin, S.; Comisso, N.; Zotti, G. Solvoconductivity of Polyconjugated Polymers: The Roles of Polymer Oxidation Degree and Solvent Electrical Permittivity. Chem. Mater. 2002, 14, 4768–4774. [Google Scholar] [CrossRef]
- Paul, S.; Amalraj, F.; Radhakrishnan, S. CO sensor based on polypyrrole functionalized with iron porphyrin. Synth. Met. 2009, 159, 1019–1023. [Google Scholar] [CrossRef]
- Paul, S.; Chavan, N.N.; Radhakrishnan, S. Polypyrrole functionalized with ferrocenyl derivative as a rapid carbon monoxide sensor. Synth. Met. 2009, 159, 415–418. [Google Scholar] [CrossRef]
- Tiwari, D.C.; Sharma, R.; Vyas, K.D.; Boopathi, M.; Singh, V.V.; Pandey, P. Electrochemical incorporation of copper phthalocyanine in conducting polypyrrole for the sensing of DMMP. Sens. Actuators B 2010, 151, 256–264. [Google Scholar] [CrossRef]
- Van, C.N.; Potje-Kamloth, K. Electrical and NOx gas sensing properties of metallophthalocyanine-doped polypyrrole/silicon heterojunctions. Thin Solid Films 2001, 392, 113–121. [Google Scholar]
- Sizun, T.; Patois, T.; Bouvet, M.; Lakard, B. Microstructured electrodeposited polypyrrole-phthalocyanine hybrid material: From morphology to ammonia sensing. J. Mater. Chem. 2012, 22, 25246–25253. [Google Scholar] [CrossRef]
- Patois, T.; Sanchez, J.B.; Berger, F.; Fievet, P.; Segut, O.; Moutarlier, V.; Bouvet, M.; Lakard, B. Elaboration of ammonia gas sensors based on electrodeposited polypyrrole—Cobalt phthalocyanine hybrid films. Talanta 2009, 117, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirsat, M.D.; Bangar, M.A.; Deshusses, M.A.; Myung, N.V.; Mulchandani, A. Polyaniline nanowires-gold nanoparticles hybrid network based chemiresistive hydrogen sulfide sensor. Appl. Phys. Lett. 2009, 94, 083502–083503. [Google Scholar] [CrossRef]
- Xie, D.; Jiang, Y.D.; Pan, W.; Li, D.; Wu, Z.M.; Li, Y.R. Fabrication and characterization of polyaniline-based gas sensor by ultra-thin film technology. Sens. Actuators B 2002, 81, 158–164. [Google Scholar] [CrossRef]
- Elizalde-Torres, J.; Hu, H.L.; Garcia-Valenzuela, A. NO2-induced optical absorbance changes in semiconductor polyaniline thin films. Sens. Actuators B 2004, 98, 218–226. [Google Scholar] [CrossRef]
- Jin, Z.; Su, Y.X.; Duan, Y.X. Development of a polyaniline-based optical ammonia sensor. Sens. Actuators B 2001, 72, 75–79. [Google Scholar] [CrossRef]
- Hong, K.H.; Oh, K.W.; Kang, T.J. Polyaniline-nylon 6 composite fabric for ammonia gas sensor. J. Appl. Polym. Sci. 2004, 92, 37–42. [Google Scholar] [CrossRef]
- Liu, H.Q.; Kameoka, J.; Czaplewski, D.A.; Craighead, H.G. Polymeric nanowire chemical sensor. Nano Lett. 2004, 4, 671–675. [Google Scholar] [CrossRef]
- Virji, S.; Huang, J.X.; Kaner, R.B.; Weiller, B.H. Polyaniline nanofiber gas sensors: Examination of response mechanisms. Nano Lett. 2004, 4, 491–496. [Google Scholar] [CrossRef]
- Hao, Q.L.; Wang, X.; Lu, L.D.; Yang, X.J.; Mirsky, V.M. Electropolymerized multilayer conducting polymers with response to gaseous hydrogen chloride. Macromol. Rapid Commun. 2005, 26, 1099–1103. [Google Scholar] [CrossRef]
- Virji, S.; Fowler, J.D.; Baker, C.O.; Huang, J.X.; Kaner, R.B.; Weiller, B.H. Polyaniline manofiber composites with metal salts: Chemical sensors for hydrogen sulfide. Small 2005, 1, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Ogura, K.; Shiigi, H.; Oho, T.; Tonosaki, T. A CO2 sensor with polymer composites operating at ordinary temperature. J. Electrochem. Soc. 2000, 147, 4351–4355. [Google Scholar] [CrossRef]
- Krivan, E.; Visy, C.; Dobay, R.; Harsanyi, G.; Berkesi, O. Irregular response of the polypyrrole films to H2S. Electroanalysis 2000, 12, 1195–1200. [Google Scholar] [CrossRef]
- Geng, L.; Wang, S.R.; Zhao, Y.Q.; Li, P.; Zhang, S.M.; Huang, W.P.; Wu, S.H. Study of the primary sensitivity of polypyrrole/γ-Fe2O3 to toxic gases. Mater. Chem. Phys. 2006, 99, 15–19. [Google Scholar] [CrossRef]
- Gustafsson, G.; Lundrom, I.; Liedberg, B.; Wu, C.R.; Inganas, O.; Wennerstom, O. The interaction between ammonia and polypyrrole. Synth. Met. 1989, 31, 163–179. [Google Scholar] [CrossRef]
- Guernion, N.; Ewen, R.J.; Pihlainen, K.; Ratcliffe, N.M.; Teare, G.C. The fabrication and characterisation of a highly sensitive polypyrrole sensor and its electrical responses to amines of differing basicity at high humidities. Synth. Met. 2002, 126, 301–310. [Google Scholar] [CrossRef]
- Kemp, N.T.; Kaiser, A.B.; Trodahl, H.J.; Chapman, B.; Buckley, R.G.; Partridge, A.C.; Foot, P.J.S. Effect of ammonia on the temperature-dependent conductivity and thermopower of polypyrrole. J. Polym. Sci. B 2006, 44, 1331–1338. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakard, B.; Carquigny, S.; Segut, O.; Patois, T.; Lakard, S. Gas Sensors Based on Electrodeposited Polymers. Metals 2015, 5, 1371-1386. https://doi.org/10.3390/met5031371
Lakard B, Carquigny S, Segut O, Patois T, Lakard S. Gas Sensors Based on Electrodeposited Polymers. Metals. 2015; 5(3):1371-1386. https://doi.org/10.3390/met5031371
Chicago/Turabian StyleLakard, Boris, Stéphanie Carquigny, Olivier Segut, Tilia Patois, and Sophie Lakard. 2015. "Gas Sensors Based on Electrodeposited Polymers" Metals 5, no. 3: 1371-1386. https://doi.org/10.3390/met5031371
APA StyleLakard, B., Carquigny, S., Segut, O., Patois, T., & Lakard, S. (2015). Gas Sensors Based on Electrodeposited Polymers. Metals, 5(3), 1371-1386. https://doi.org/10.3390/met5031371