3.4. Phase Evolution and Alloying Element Distribution during Partial Remelting of Compressed 7075 Alloy
A close examination of the micrographs in
Figure 3 reveals that the volume fractions of precipitates in the microstructure reduce with the rise of the isothermal holding time. This is clearly confirmed by increasing the brightness of the recrystallized solid grains. XRD results shown in
Figure 5 also confirm this phenomenon. The X-ray diffraction pattern of the 20% compressed sample prior to the isothermal treatment, indicated that the microstructure consists of α-Al phase and η, S, Al
6(Cu,Fe), Al
7Cu
2Fe, and Mg
2Si intermetallic phases, which is similar to the as-received sample (
Figure 1). According to the X-ray diffraction patterns of samples heat treated at 600 °C for 5 to 35 min, it is clear that the intensity of η, S, and Mg
2Si peaks decrease with the increase of the holding time. The diffraction peaks of the 20% compressed sample are shown by symbols and the corresponding peaks in each case are specified by arrows for the other samples in
Figure 5. The diffraction peaks of η, S, and Mg
2Si phases almost disappeared after heating for 25 min. However, some diffraction peaks of Fe-rich intermetallic phases (Al
7Cu
2Fe and Al
6(Cu, Fe)) show little change with prolonging of the holding time. Considering the sample heated for 35 min, the presence of Al
7Cu
2Fe and Al
6(Cu, Fe) phases was distinguishable. However, no evidence of η, S, and Mg
2Si phases was observed. This illustrates that Fe-rich secondary particles cannot be dissolved into the matrix for the semi-solid treatment conditions used in the present study.
Previous work reported that the melting temperature of η-phase in 7xxx alloys is 475–480 °C [
26,
27]. This is fairly well correlated with the first peak in the DSC curve of the starting material with an enthalpy of 2.7 J/g in
Figure 1. The solution temperatures of S and Mg
2Si phases are 490–501 °C [
28,
29] and 478–525 °C [
30], respectively, depending on the Mg content of the alloy. Considering the XRD results (
Figure 5) and solution temperature of intermetallic precipitates, it can be suggested that the dissolving of intermetallic particles commences with melting of η precipitates after heating for 10 min through the reaction of α-Al + η → L. Following the increase of the heating time, dissolving of S and Mg
2Si intermetallic particles also occurs through the phase transformation of α-Al + S + Mg
2Si → L. These precipitates are fully dissolved after isothermal heating for 25 min.
The Fe-rich intermetallic precipitates have higher solution temperatures and are usually stable over 600 °C [
31]. Microstructural observations revealed that the Fe-rich intermetallic particles, shown in
Figure 6, precipitate at grain boundaries during partial remelting. Precipitation of these particles at the grain boundaries has a great influence on pinning of the grain boundaries movement. Therefore, it may cause the retardation of the particle growth and coarsening during the isothermal treatment. This is discussed in more detail in
Section 3.5.
It can be concluded from the above discussion that the distribution of alloying elements in the microstructure is significantly changed during the isothermal treatment and solution of the constituent particles. Segregation and distribution of the alloying elements has a great influence on the microstructural evolution during semi-solid treatment. Moreover, this affects the mechanical properties of the subsequent thixoformed parts [
32,
33,
34].
Figure 6 shows the SEM images of the compressed samples heat treated at 600 °C (
Figure 6a,b) and 610 °C (
Figure 6c,d) for 25 min. The EDS analysis results of the marked areas (A to D) in
Figure 6b are shown in
Figure 7. Intense concentration of Cu at the grain boundaries (point A) was observed. In addition, Si intensely segregated at other regions of the grain boundaries (point B). The entrapped liquid droplets within the solid grains (point C) also showed high concentration of Cu. Although the segregation of Cu at the grain boundaries and within the liquid droplets has been reported in the literature [
35,
36], the segregation of Si is an unreported phenomenon in the semi-solid microstructure of 7075 Al alloy. Solid grains are depleted from Cu as a consequence of the segregation of Cu at the grain boundaries. This results in a rise in the solidus temperature in these regions and a decline of the temperature at the grain boundaries. Finally, chemical composition approaches Al-Cu eutectic composition which facilitates grain boundary melting. The segregation of Si at other regions of the grain boundaries derives grain boundary chemistry closer to Al-Si eutectic composition. Therefore, it can be concluded that the formation of low melting phases at grain boundaries is mainly influenced by the high content of Cu and Si.
Grain boundary regions with low solidus temperature remelt during the isothermal heating. Thus, a eutectic liquid film surrounds the solid grains. Melting of solid grains occurs on further increasing the heating temperature. However, it should be noted that the segregation of Cu and Si at the grain boundaries improves on raising the heating temperature due to more intense atomic diffusion. The EDS analysis of the precipitate particles at the grain boundaries (point D) in
Figure 6 (
Figure 7d) demonstrates Fe-rich intermetallic phases. This is also confirmed by XRD patterns in
Figure 5. As discussed earlier, these particles cannot be dissolved at 600 °C and appear mainly as square particles at the grain boundaries in the semi-solid microstructure. The results of the microstructural investigations in this research showed that the amount of these particles reduce on increasing the isothermal heating temperature to 620 °C and isothermal heating for longer times (>15 min).
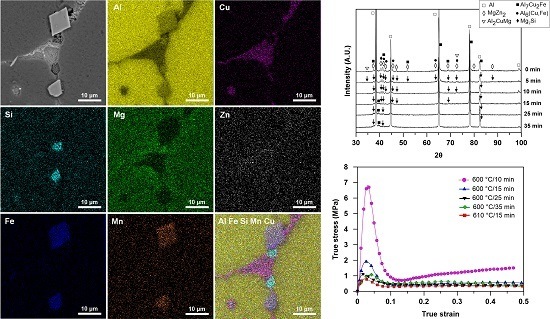
Figure 8 shows the EDS mapping of the main alloying elements of the strained samples heat treated at 600 °C for 25 min. Considerable segregation of Cu and Si at the grain boundaries was also confirmed by the EDS mapping analysis of Cu and Si (
Figure 8c,d). In addition, an appreciable depletion of Mg was observed at the grain boundaries (
Figure 8e). However, as it can be observed from the EDS analysis results in
Figure 7 and the EDS mapping of Mg in
Figure 8e, the Al-Si eutectics have a higher content of Mg compared with the Al-Cu eutectics. There was no appreciable change in Zn content at various point of the microstructure (
Figure 8f). This is in contrast to the result of researches conducted by Bolouri
et al. [
35] and Shim
et al. [
37] who reported the segregation of Zn at the grain boundaries of a SIMA processed 7075 alloy and semi-solid Al-Zn-Mg alloy prepared by the cooling plate method, respectively. Atkinson
et al. [
18] also indicated that Zn and Mg are enriched at the grain boundaries of RAP processed 7075 alloy. Moreover, as it can be observed in
Figure 8g,h, the grain boundary precipitates contain a high amount of Fe and Mn. As the EDS analysis revealed (
Figure 7c,d), these precipitates are attributed to Fe-rich intermetallic particles. An overview of the alloying elements distribution in the semi-solid microstructure of 7075 Al alloy can be observed in
Figure 8i.
Figure 9a–e shows SEM images of compressed samples heat treated at 600 °C for 5–35 min. Variations of the main alloying elements of 7075 alloy (
i.e. Cu, Zn, and Mg) at the grain boundaries and grain centers in these samples are also demonstrated in
Figure 9f. The EDS analysis results of the grain centers and grain boundaries (
Figure 9f), showed that the Cu concentration reduces within the solid grains on increasing the isothermal holding time at 600 °C. In other words, Cu segregation rises at grain boundaries with increasing holding time. Mg concentration declined at the grain boundaries on raising the holding time and no significant change was observed in Zn concentration at various parts of the microstructure.
Considering that (i) Zn, Mg, Si, and Cu-bearing intermetallic precipitates (η, S and Mg
2Si) are dissolved gradually on prolonging the holding time according to XRD patterns of the SIMA processed samples (
Figure 5), (ii) Zn and Mg have higher solubility in Al compared with Cu and Si according to the Al-Zn, Al-Mg, Al-Cu and Al-Si binary phase diagrams [
38], and (iii) no distinct peak for these elements appeared in the XRD patterns (
Figure 5), it can be concluded that Zn and Mg elements are easily dissolved in α-Al, while Cu and Si significantly segregate at the grain boundaries. Since, Zn has a smaller atomic radius relative to aluminum (0.133 nm compare to 0.143 nm) [
39], it can be concluded that dissolving Zn in the aluminum crystal lattice leads to reduction of the lattice parameter of aluminum. However, dissolving Mg in Al, as a result of its greater atomic radius, can result in increase of the lattice parameter of aluminum. The shifting of the Al main peaks in XRD patterns (
Figure 5) to higher angles, as shown in
Figure 10a for (111) reflection of the aluminum matrix, revealed that the lattice parameter of Al decreases on prolonging the heating time (
Figure 10b) based on Bragg’s equation (
nλ = 2
dsinθ). It seems that dissolving Zn in Al is more effective than Mg. This may be as a result of the higher Zn content of 7075 alloy. According to
Figure 10b, the lattice parameter is gently reduced with prolonging holding time to 15 min and then with further extension of the holding time to 25 min, the lattice parameter precipitously declines. Finally, further increasing of the heating time results in a gentle reduction of the lattice parameter. These observations clearly correlate with the XRD results in
Figure 5. According to the XRD patterns, great amounts of η precipitates dissolve during the isothermal heating of the sample for 25 min. This results in dissolving of a higher amount of Zn atoms in the aluminum lattice and therefore leads to a significant decline of the lattice parameter.
3.5. Effects of Isothermal Holding Temperature and Time on the Semi-Solid Microstructure
Figure 11 shows the quenched microstructures of 7075 samples which compressed 20% and heated at 600, 610, and 620 °C for different times. A close examination of the micrographs in
Figure 11 reveals that the liquid fraction and the sphericity of solid grains become greater on raising the isothermal holding temperature and time. Another fact that can be observed in the micrographs is the reduction of the number of entrapped liquid droplets within the grains and increase of their size on extending the heating temperature and time.
Variations of the average grain size and shape factor of strained samples heated at different temperatures and times are shown in
Figure 12. As expected, the average grain size and shape factor become greater with increase of the holding temperature and time. However, it is worth noting that no significant variation of the average grain size of samples heated for 15 min on raising the holding temperatures from 600 to 620 °C is observed. Disintegration of solid particles owing to liquid phase penetration between the solid grains occurred during the primary heating time. This is due to the fact that a large number of solid grains with the same crystallographic orientations make contact with each other. It seems that the mechanism of particle disintegration is in equilibrium with the coalescence mechanism at longer heating times up to 15 min. Therefore, the solid grain sizes show no appreciable variation. In contrast, with further extension of holding times at various heating temperatures, the liquid film thickness between the solid grains and the liquid fraction becomes greater. Consequently, the Ostwald ripening mechanism can be considered as a dominant mechanism in the solid grain coarsening process.
Figure 3e,f shows separated spherical grains with large entrapped liquid droplets which is a fact that supports the discussion above.
Results of research conducted by Vaneetveld
et al. [
40] showed that the semi-solid feedstock with high solid fraction (
fs ≈ 0.84) shows better thixotropic behavior during semi-solid forming and also significantly prevents the formation of some defects such as porosity and shrinkage during the forming process. According to the solid fraction
versus temperature curve in
Figure 2b, the solid fraction is relatively low at 620 °C (
fs ≈ 0.55). Thus, heating samples at such a temperature leads to undesirable coarsening of the solid grains. In addition, the solid fraction is more sensitive to temperature variations at 620 °C, when compared to the lower temperatures in which control of the semi-solid forming parameters is difficult. Consequently, the semi-solid microstructure obtained by isothermal heating at 620 °C is inappropriate for thixotropic applications. However, high solid fractions were observed following heating at temperatures of 600 and 610 °C. The solid volume fractions at 600 and 610 °C were 0.8 and 0.7, respectively (
Figure 2b). Average grain size smaller than 75 μm and shape factor greater than 0.7 were obtained for strained samples heated at 600 and 610 °C for 25 min. Therefore, the isothermal heating temperature range of 600 to 610 °C and holding time of 25 min can be considered the optimum semi-solid treatment condition.
The coarsening kinetics can be expressed by the Lifshitz-Slyozov-Wagner (LSW) relationship [
41,
42]:
where
D and
D0 are the final and initial grain sizes, respectively,
t is the isothermal holding time,
k is the coarsening rate constant and
n is the power exponent. It has been found that
n is 3 for volume diffusion controlled systems in the semi-solid state [
12].
In the present research, the coarsening rate constant (
k) was calculated by fitting a power relationship to the experimental results.
Figure 13 shows the cube of grain size variations
versus isothermal holding time for compressed samples heated at 600, 610, and 620 °C, where
D0 is the average grain size when the holding time is 15 min. The regression coefficients of the fitted equations are close to 1. Thus, it can be concluded that the coarsening kinetics of solid particles during the isothermal heating of deformed 7075 samples at the semi-solid temperature range are fairly well correlated with the LSW equation. The values of the coarsening rate constant
k for the samples are summarized in
Table 3. The main characteristics of the variation of
k values when the temperature was decreased from 620 to 600 °C are as follows; (i) the
k value significantly reduced on decreasing the temperature from 620 to 610 °C; (ii) this was followed by a slight increase at 600 °C.
According to the Doherty theorem [
32], the coarsening rate accelerates with rising solid fraction (
fs) for
fs higher than 0.6. However, the research conducted by Manson-Whitton
et al. [
41] showed opposite results for higher solid fractions (
fs ≥ 0.7) in the case of spray formed Al-4% Cu. Therefore, they proposed a modified model considering the solid-solid contacts effect during coarsening and a transition solid fraction (
fs ≈ 0.7). For the solid fractions greater than the transition value, the
k value decreased with an increase in the solid fraction. However, for the solid fraction lower than the transition value, the
k value increased on increasing the solid fraction. Considering the holding temperatures that correspond to the calculated solid fractions (
Figure 2b), for solid fractions greater than 0.7 (at 610 °C) and the transition value of 0.8 (at 600 °C), the coarsening process of solid grains is similar to the model proposed by Manson-Whitton
et al. [
41]. On the other hand, an unexpected increase in
k values is observed for solid fractions lower than 0.7. This can be attributed to (i) further increase of the atomic diffusion and more effective Ostwald ripening mechanism and (ii) a less retarding effect of the precipitate particles (Fe-rich intermetallic particles) for a holding temperature up to 620 °C. These particles bring about convoluting grain boundaries as is marked by arrows in
Figure 14, suggesting pinning and retardation of the grain boundary liquid film migration during the solid grains coarsening. The effect of the presence of intermetallic precipitates at the grain boundaries on the coarsening rate of aluminum alloys has also been reported by Manson-Whitton
et al. [
41] and de Freitas
et al. [
43]. The results obtained in the present research indicate that Fe-rich precipitates mainly dissolve or become smaller than the thickness of the liquid film on raising the heating temperature up to 620 °C. Thus, the movement of grain boundaries can easily occur and leads to greater values of the coarsening rate constant.
3.6. Semi-Solid Deformation Behavior
Figure 15 shows a typical load-displacement curve obtained from the hot compression experiment. As it can be observed, the load rises to a maximum (
Lmax) and then decreases to a minimum value (
Lmin). The thixotropic flow behavior consisted of a transient regime and a steady state regime, as shown in
Figure 15. Semi-solid alloys with an equiaxed microstructure are considered as deformable semi-cohesive granular solids saturated with liquid [
44]. The solid grains are partially interconnected by unwetted grain boundaries and liquid fills the interstitial spaces. Large deformation results in the destruction of some solid grain boundary bonds to allow grains to move freely and rearrange through sliding and rolling. The cohesion at the grain level reflects the strength of unwetted grain boundaries and results in a flow resistance. With further deformation breakdown the solid agglomerates, the load (stress) decreases to a steady state level.
Tzimas and Zavaliangos [
44] suggested that the flow resistance of semi-solid alloys at high solid volume fraction is controlled by four mechanisms: (i) elastoplastic deformation at grain contacts, (ii) destruction of cohesive bonds between solid grains, (iii) resistance to the flow of liquid relative to the solid, and (iv) resistance to grain rearrangement. Mechanism (i) is dominant in the rising part of the flow curve and mechanism (ii) results in the breakdown of solid particles and a significant drop in the load value. Mechanisms (iii) and (iv) are dominant in the second stage, and their relative contribution to the overall resistance to flow depends on the volume fraction of solid.
The stress-strain curves obtained from the SIMA processed 7075 samples with different initial microstructures after hot compression at 600 °C (for the SIMA samples isothermally treated at 600 °C for 10–35 min) and 610 °C (for the SIMA sample isothermally treated at 610 °C for 15 min) are shown in
Figure 16. The stress exhibits a maximum value at a relatively low strain in all samples. The strain at the peak stress varies between 0.01 and 0.04. The peak stress then drops to a steady state value, typically at a strain of 0.1–0.18. The stress at the steady state shows no significant change with strain.
Figure 17 shows the variation of the peak and steady state stresses for the SIMA processed samples with different initial microstructures. As is evident from
Figure 16 and
Figure 17, the sample semi-solid treated at 600 °C for 10 min exhibits the highest peak and steady state stresses. On increasing the isothermal holding time to 25 min at 600 °C, which results in a coarser and more spherical solid grains in the initial microstructure (see
Figure 12), both the peak and steady state stresses decreased significantly. The peak stress showed a slight increase while a considerable increase in the steady state stress was observed with further increase of the holding time to 35 min in the SIMA process. Moreover, the increase of the deformation temperature had a great influence on the stress-strain curve and resulted in a decrease of thixotropic strength, as is observed for the sample semi-solid treated at 610 °C for 15 min.
The descending trend of the thixotropic strength (decreasing of the peak stress) with increase of the isothermal holding time in the SIMA process can be attributed to the geometry of the solid grains. As was previously described, the deformation of samples with equiaxed microstructure mainly occurs by grain rearrangement through sliding and rolling. However, the microstructures consisting of non-equiaxed and elongated grains are deformed by plastic deformation due to which the relative movement among solid grains is constrained.
Figure 18 shows the hot compressed microstructure of SIMA processed samples with near equiaxed and globular initial microstructure. The microstructure of various regions (1 to 3 in
Figure 18a) of the hot compressed SIMA sample processed at 600 °C for 10 min (see
Figure 3c) revealed that this sample cannot be deformed by grain rearrangement due to the significant geometric interference of the solid grains which results in plastic deformation of the grains (
Figure 18b–d). As is observed in
Figure 18b–d, the solid grains are severely deformed and elongated in the direction of compression which results in a textured microstructure. This leads to a much higher resistance to flow compared to samples with a completely spheroidized microstructure. On prolonging the isothermal heating time, the sphericity of solid grains increases and the interconnection decreases (
Figure 3) which results in easier movement and sliding during deformation and reduction of flow resistance. The high flow stress of the SIMA sample heated for 35 min can be ascribed to the coarsened initial microstructure with an average grain size of 85 μm. According to Clarke [
45], an increase in the particle size leads to an increase in the apparent viscosity of the semi-solid slurry. Therefore, a sample with coarsened initial microstructure possesses a more viscous flow and requires higher loads for thixoforming.
Figure 18e,f shows the microstructure of the center region (region 2 in
Figure 18a) of the hot compressed semi-solid samples prepared by heating at 600 °C for 25 and 35 min, respectively. After compression, no evidence of the plastic deformation of the solid grains was observed in the microstructure of the samples. There was no difference in the microstructure of the center regions and edge regions, which indicated that the solid and the liquid flowed together during the compression. However, it is worth mentioning that the liquid fraction in the edge regions was slightly high, which was due to the liquid phase being squeezed out during the deformation. The microstructural observations also revealed that the solid grains do not experience plastic deformation in the SIMA samples prepared by heating at 600 and 610 °C for 15 min. According to Chen and Tsao [
46], the plastic deformation of solid particles (PDS), sliding between the solid particles (SS), liquid flow (LF), and the flow of liquid incorporating solid particles (FLS) are four dominant mechanisms controlling deformation of alloys in the semi-solid state. The two former mechanisms are active when the solid particles are in contact with each other and the other two mechanisms are dominant when the solid particles are surrounded by the liquid phase. Therefore, it can be deduced that the sample with near equiaxed initial microstructure deform through the mechanism of PDS. In the case of globular initial microstructures, the SS and FLS mechanisms are dominant, however, the effectiveness of the SS mechanism may dwindle and the FLS mechanism is reinforced on prolonging the isothermal holding temperature and time due to the decrease of the interconnection between the solid grains.
The close examination of the hot compressed microstructure of different samples (
Figure 18) also reveals that the microstructure of the SIMA processed sample with near equiaxed and elongated initial grains contains a higher amount of porosity, especially in regions 2 and 3, (shown by arrows in
Figure 18c,d) compared to the samples with globular microstructure. In the sample with near equiaxed initial microstructure, the low amount of liquid content leads to higher viscosity, which may prevent the homogenous flow of the liquid and solid particles during deformation. Therefore, this sample shows a high amount of porosity in the microstructure. In addition, as shown by dotted ovals in
Figure 18c, recrystallization occurs in some parts of the center region in the sample with an incomplete spheroidized initial microstructure during hot compression. It is believed that this recrystallization may occur dynamically as a result of higher induced strain in the center regions compared to the edge regions during hot compression.