Effects of Cryogenic Forging and Anodization on the Mechanical Properties and Corrosion Resistance of AA6066–T6 Aluminum Alloys
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Tensile and Fatigue Tests
2.3. X-ray Tests
2.4. Anodization Process
3. Results and Discussion
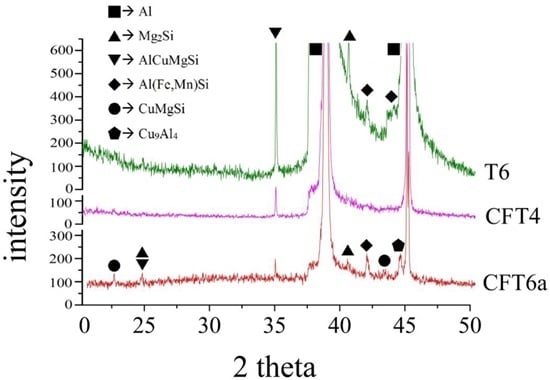
3.1. Microstructure Observation and Tensile Properties
3.2. Fatigue and Corrosion Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, Y.M.; Ma, E.; Chen, M.W. Enhanced tensile ductility and toughness in nanostructured Cu. Appl. Phys. Lett. 2002, 80, 2395–2397. [Google Scholar] [CrossRef]
- Rangaraju, N.; Raghuram, T.; Krishna, B.V.; Rao, K.P.; Venugopal, P. Effect of cryo-rolling and annealing on microstructure and properties of commercially pure aluminium. Mater. Sci. Eng. A Struct. 2005, 398, 246–251. [Google Scholar] [CrossRef]
- Chen, Y.J.; Roven, H.J.; Gireesh, S.S.; Skaret, P.C.; Hjelen, J. Quantitative study of grain refinement in Al–Mg alloy processed by equal channel angular pressing at cryogenic temperature. Mater. Lett. 2011, 65, 3472–3475. [Google Scholar] [CrossRef]
- Lee, Y.B.; Shin, D.H.; Park, K.T.; Nam, W.J. Effect of annealing temperature on microstructures and mechanical properties of a 5083 Al alloy deformed at cryogenic temperature. Scr. Mater. 2004, 51, 355–359. [Google Scholar] [CrossRef]
- Panigrahi, S.K.; Jayagathan, R. A study on the mechanical properties of cryorolled Al–Mg–Si alloy. Mater. Sci. Eng. A Struct. 2008, 480, 299–305. [Google Scholar] [CrossRef]
- Panigrahi, S.K.; Jayagathan, R.; Chawla, V. Effect of cryorolling on microstructure of Al–Mg–Si alloy. Mater. Lett. 2008, 62, 2626–2639. [Google Scholar] [CrossRef]
- Panigrahi, S.K.; Jayagathan, R. Development of ultrafine-grained Al 6063 alloy by cryorolling with the optimized initial heat treatment conditions. Mater. Des. 2011, 32, 2172–2180. [Google Scholar] [CrossRef]
- Krishna, K.G.; Sivaprasad, K.; Venkateswarlu, K.; Kumar, K.C.H. Microstructural evolution and aging behavior of cryorolled Al–4Zn–2Mg alloy. Mater. Sci. Eng. A Struct. 2012, 535, 129–135. [Google Scholar] [CrossRef]
- Das, P.; Jayaganthan, R.; Singh, I.V. Tensile and impact-toughness behavior of cryorolled Al 7075 alloy. Mater. Des. 2011, 32, 1298–1305. [Google Scholar] [CrossRef]
- Panigrahi, S.K.; Jayaganthan, R. Effect of ageing on microstructure and mechanical properties of bulk, cryorolled, and room temperature rolled Al 7075 alloy. J. Alloy. Compd. 2011, 509, 9609–9016. [Google Scholar] [CrossRef]
- Yin, J.; Lu, J.; Ma, H.; Zhang, P. Nanostructural formation of fine grained aluminum alloy by severe plastic deformation at cryogenic temperature. J. Mater. Sci. 2004, 39, 2851–2854. [Google Scholar] [CrossRef]
- Krishna, K.G.; Sivaprasad, K.; Narayanana, T.S.N.S.; Kumar, K.C. Localized corrosion of an ultrafine grained Al–4Zn–2Mg alloy produced by cryorolling. Corros. Sci. 2012, 60, 82–89. [Google Scholar] [CrossRef]
- Shanmugasundaram, T.; Murty, B.S.; Sarma, V.S. Development of ultrafine grained high strength Al–Cu alloy by cryorolling. Scr. Mater. 2006, 54, 2013–2017. [Google Scholar] [CrossRef]
- Rao, P.N.; Jayaganthan, R. Effects of warm rolling and ageing after cryogenic rolling on mechanical properties and microstructure of Al 6061 alloy. Mater. Des. 2012, 39, 226–233. [Google Scholar]
- Huang, Y.S.; Shih, T.S.; Chou, J.H. Electrochemical behavior of anodized AA7075-T73 alloys affected by matrix structures. Appl. Surf. Sci. 2013, 283, 249–257. [Google Scholar] [CrossRef]
- ASTM B557-15, Standard Test Methods for Tension Testing Wrought and Cast Aluminum- and Magnesium-Alloy Products; ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- JIS Z2274, Method of Rotating Bending Fatigue Testing of Metals; Japanese Standards Association: Tokyo, Japan, 1978.
- Shih, T.S.; Lee, T.H.; Jhou, Y.J. The effects of anodization treatment on the microstructure and fatigue behavior of 7075-T73 aluminum alloy. Mater. Trans. 2014, 55, 1280–1285. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.Y.; Paik, K.W.; Koh, K.W.; Won, J.; Choe, S.; Lee, J.; Moon, J.T.; Park, Y.J. Effects of Cu/Al intermetallic compound (IMC) on copper wire and aluminum pad bondability. IEEE Trans. Pack. Technol. 2003, 26, 367–374. [Google Scholar]
- Du, Y.; Chang, Y.A.; Huang, B.; Gong, W.; Jin, Z.; Xu, H.; Yuan, Z.; Liu, Y.; He, Y.; Xie, F.Y. Diffusion coefficients of some solutes in fcc and liquid Al: Critical evaluation and correlation. Mater. Sci. Eng. A Struct. 2003, 363, 140–151. [Google Scholar] [CrossRef]
- Matsuda, K.; Teguri, D.; Sato, T.; Uetani, Y.; Ikeno, S. Cu Segregation around Metastable Phase in Al–Mg–Si Alloy with Cu. Mater. Trans. 2007, 48, 967–974. [Google Scholar] [CrossRef]
- Svenningsen, G.; Larsen, M.H.; Walmsley, J.C.; Nordlien, J.H.; Nisancioglu, K. Effect of artificial aging on intergranular corrosion of extruded AlMgSi alloy with small Cu content. Corros. Sci. 2006, 48, 1528–1543. [Google Scholar] [CrossRef]
- Vieira, A.C.; Pinto, A.M.; Rocha, L.A.; Mischler, S. Effect of Al2Cu precipitates size and mass transport on the polarisation behaviour of age-hardened Al–Si–Cu–Mg alloys in 0.05 M NaCl. Electrochim. Acta 2011, 56, 3821–3828. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.J.; Muggerud, A.M.F.; Olsen, A.; Furu, T. Precipitation of partially coherent α-Al(Mn,Fe)Si dispersoids and their strengthening effect in AA 3003 alloy. Acta Mater. 2012, 60, 1004–1014. [Google Scholar] [CrossRef]
- Cabibbo, M. Microstructure strengthening mechanisms in different equal channel angular pressed aluminum alloys. Mater. Sci. Eng. A Struct. 2013, 560, 413–432. [Google Scholar] [CrossRef]
- Zhaia, T.; Jianga, X.P.; Lia, J.X.; Garratt, M.; Bray, G.H. The grain boundary geometry for optimum resistance to growth of short fatigue cracks in high strength Al-alloys. Int. J. Fatigue 2005, 27, 1202–1209. [Google Scholar] [CrossRef]
- Huang, Y.S.; Shih, T.S.; Wu, C.E. Electrochemical behavior of anodized AA6063-T6 alloys affected by matrix structures. Appl. Surf. Sci. 2013, 264, 410–418. [Google Scholar] [CrossRef]
- Shih, T.S.; Chiu, Y.W. Corrosion resistance and high-cycle fatigue strength of anodized/sealed AA7050 and AA7075 alloys. Appl. Surf. Sci. 2015, 351, 997–1003. [Google Scholar] [CrossRef]











| Sample | Tensile Strengths | Vickers Hardness (HV) | ||
|---|---|---|---|---|
| UTS (MPa) | YS (MPa) | Elongation (%) | ||
| T6 | 460 (0.5) | 438 (1.4) | 10.6 (1.7) | 133.6 (2.9) |
| CFT6a | 431 (6.6) | 394 (9.2) | 14.2 (0.2) | 136.6 (1.7) |
| CFT6b | 435 (5.4) | 404 (10.1) | 13.4 (0.9) | 137.1 (2.9) |
| Sample | Second Phase (Coarse Precipitates) Counts/mm2 | Max. Diameter of Particle, μm | |||
|---|---|---|---|---|---|
| 1–10 | 11–20 | >20 | Total Count | ||
| T6 | 838 | 25 | 0 | 864 | 17 |
| CFT6a | 719 | 15 | 0 | 734 | 17 |
| Sample | Surface Roughness (μm) |
|---|---|
| T6; CFT6a | 0.07 (0.04) |
| T6-A | 0.16 (0.03) |
| CFT6a-A | 0.13 (0.06) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, T.-S.; Yong, H.-S.; Hsu, W.-N. Effects of Cryogenic Forging and Anodization on the Mechanical Properties and Corrosion Resistance of AA6066–T6 Aluminum Alloys. Metals 2016, 6, 51. https://doi.org/10.3390/met6030051
Shih T-S, Yong H-S, Hsu W-N. Effects of Cryogenic Forging and Anodization on the Mechanical Properties and Corrosion Resistance of AA6066–T6 Aluminum Alloys. Metals. 2016; 6(3):51. https://doi.org/10.3390/met6030051
Chicago/Turabian StyleShih, Teng-Shih, Hwa-Sheng Yong, and Wen-Nong Hsu. 2016. "Effects of Cryogenic Forging and Anodization on the Mechanical Properties and Corrosion Resistance of AA6066–T6 Aluminum Alloys" Metals 6, no. 3: 51. https://doi.org/10.3390/met6030051
APA StyleShih, T. -S., Yong, H. -S., & Hsu, W. -N. (2016). Effects of Cryogenic Forging and Anodization on the Mechanical Properties and Corrosion Resistance of AA6066–T6 Aluminum Alloys. Metals, 6(3), 51. https://doi.org/10.3390/met6030051