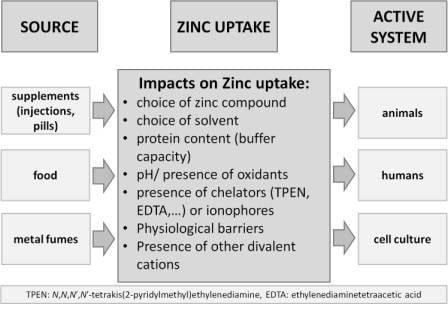
Parameters Influencing Zinc in Experimental Systems in Vivo and in Vitro
Abstract
:1. Introduction
2. Considerations When Working with Zinc
3. Zinc in Cell Culture Experiments
4. Measurement of Zinc
4.1. Measurement of Total Zinc
4.2. Measurement of the Zinc Status
4.3. Measurement of Free Zn2+
5. Influences on Zinc Bioavailability and Functions in Experimental Systems in Vivo
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, W.J.; Zhao, C.Y.; Zheng, T.L. Comparison of zinc contents in human serum and plasma. Clin. Chim. Acta 1986, 155, 185–187. [Google Scholar] [PubMed]
- Rink, L.; Gabriel, P. Zinc and the immune system. Proc. Nutr. Soc. 2000, 59, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Hebel, S.; Engelhardt, G.; Rink, L. The biochemical effects of extracellular Zn2+ and other metal ions are severely affected by their speciation in cell culture media. Metallomics 2015, 7, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Colvin, R.A.; Holmes, W.R.; Fontaine, C.P.; Maret, W. Cytosolic zinc buffering and muffling: Their role in intracellular zinc homeostasis. Metallomics 2010, 2, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Krezel, A.; Maret, W. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J. Biol. Inorg. Chem. 2006, 11, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Noh, S.J.; Pronto, J.R.; Jeong, Y.J.; Kim, H.K.; Song, I.S.; Xu, Z.; Kwon, H.Y.; Kang, S.C.; Sohn, E.H.; et al. The critical roles of zinc: Beyond impact on myocardial signaling. Korean J. Physiol. Pharmacol. 2015, 19, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Vallee, B.L.; Falchuk, K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [PubMed]
- Kloubert, V.; Rink, L. Zinc as a micronutrient and its preventive role of oxidative damage in cells. Food Funct. 2015, 6, 3195–3204. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Rink, L. Functional significance of zinc-related signaling pathways in immune cells. Annu. Rev. Nutr. 2009, 29, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Zinc in the biosciences. Metallomics 2014, 6, 1174. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Analyzing free zinc(II) ion concentrations in cell biology with fluorescent chelating molecules. Metallomics 2015, 7, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Ackland, M.L.; Michalczyk, A. Zinc deficiency and its inherited disorders—A review. Genes Nutr. 2006, 1, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Hirano, T. Intracellular zinc homeostasis and zinc signaling. Cancer Sci. 2008, 99, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Mocchegiani, E.; Rink, L. Correlation between zinc status and immune function in the elderly. Biogerontology 2006, 7, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.M.; Fekete, K.; Decsi, T. Methods of assessment of zinc status in humans: A systematic review. Am. J. Clin. Nutr. 2009, 89, 2040s–2051s. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Hebel, S.; Engelhardt, G.; Rink, L. Flow cytometric measurement of labile zinc in peripheral blood mononuclear cells. Anal. Biochem. 2006, 352, 222–230. [Google Scholar] [CrossRef] [PubMed]
- National center for biotechnology information. Pubchem compound database; cid = 5727. Available online: https://pubchem.Ncbi.Nlm.Nih.Gov/compound/5727 (accessed on 21 December 2015).
- National center for biotechnology information. Pubchem compound database; cid = 24424. Available online: https://pubchem.Ncbi.Nlm.Nih.Gov/compound/24424 (accessed on 21 December 2015).
- National center for biotechnology information. Pubchem compound database; cid = 14806. Available online: https://pubchem.Ncbi.Nlm.Nih.Gov/compound/14806 (accessed on 21 December 2015).
- Zinc phosphate safety data sheet. Available online: Http://www.Sigmaaldrich.Com/msds/msds/displaymsdspage.Do?Country=de&language=en-generic&productnumber=ps803&brand=supelco&pagetogotourl=http%3a%2f%2fwww.Sigmaaldrich.Com%2fcatalog%2fproduct%2fsupelco%2fps803%3flang%3dde (accessed on 21 December 2015).
- Haase, H.; Overbeck, S.; Rink, L. Zinc supplementation for the treatment or prevention of disease: Current status and future perspectives. Exp. Gerontol. 2008, 43, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.E.; Lomeda, R.A.; Ryu, S.H.; Lee, J.H.; Beattie, J.H.; Kwun, I.S. Cellular Zn depletion by metal ion chelators (TPEN, DTPA and chelex resin) and its application to osteoblastic MC3T3-E1 cells. Nutr. Res. Pract. 2007, 1, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Bozym, R.A.; Thompson, R.B.; Stoddard, A.K.; Fierke, C.A. Measuring picomolar intracellular exchangeable zinc in PC-12 cells using a ratiometric fluorescence biosensor. ACS Chem. Biol. 2006, 1, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Mayer, L.S.; Uciechowski, P.; Meyer, S.; Schwerdtle, T.; Rink, L.; Haase, H. Differential impact of zinc deficiency on phagocytosis, oxidative burst, and production of pro-inflammatory cytokines by human monocytes. Metallomics 2014, 6, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, T.; Tawaramoto, M.; Kennedy, D.O.; Kojima, A.; Matsui-Yuasa, I. Apoptosis induced by chelation of intracellular zinc is associated with depletion of cellular reduced glutathione level in rat hepatocytes. Chem.-Biol. Interact. 2000, 125, 151–163. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Suh, S.W.; Koh, J.Y.; Cha, Y.K.; Thompson, R.B.; LaBuda, C.J.; Balaji, R.V.; Cuajungco, M.P. Depletion of intracellular zinc from neurons by use of an extracellular chelator in vivo and in vitro. J. Histochem. Cytochem. 2002, 50, 1659–1662. [Google Scholar] [CrossRef] [PubMed]
- Daaboul, D.; Rosenkranz, E.; Uciechowski, P.; Rink, L. Repletion of zinc in zinc-deficient cells strongly up-regulates IL-1beta-induced IL-2 production in T-cells. Metallomics 2012, 4, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.J.; Bradwell, A.R. Identification of the serum binding proteins for iron, zinc, cadmium, nickel, and calcium. Clin. Chem. 1983, 29, 629–633. [Google Scholar] [PubMed]
- Foote, J.W.; Delves, H.T. Albumin bound and alpha 2-macroglobulin bound zinc concentrations in the sera of healthy adults. J. Clin. Pathol. 1984, 37, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Driessen, C.; Hirv, K.; Wellinghausen, N.; Kirchner, H.; Rink, L. Influence of serum on zinc, toxic shock syndrome toxin-1, and lipopolysaccharide-induced production of IFN-gamma and IL-1 beta by human mononuclear cells. J. Leukoc. Biol. 1995, 57, 904–908. [Google Scholar] [PubMed]
- Oteiza, P.I. Zinc and the modulation of redox homeostasis. Free Radic. Biol. Med. 2012, 53, 1748–1759. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Maret, W. Aldehydes release zinc from proteins. A pathway from oxidative stress/lipid peroxidation to cellular functions of zinc. FEBS J. 2006, 273, 4300–4310. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; Hogstrand, C.; Maret, W. Picomolar concentrations of free zinc(II) ions regulate receptor protein-tyrosine phosphatase beta activity. J. Biol. Chem. 2012, 287, 9322–9326. [Google Scholar] [CrossRef] [PubMed]
- Maret, W.; Jacob, C.; Vallee, B.L.; Fischer, E.H. Inhibitory sites in enzymes: Zinc removal and reactivation by thionein. Proc. Natl. Acad. Sci. USA 1999, 96, 1936–1940. [Google Scholar] [CrossRef] [PubMed]
- Kindness, A.; Sekaran, C.N.; Feldmann, J. Two-dimensional mapping of copper and zinc in liver sections by laser ablation-inductively coupled plasma mass spectrometry. Clin. Chem. 2003, 49, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Krebs, N.F.; Miller, L.V.; Hambidge, K.M. Zinc deficiency in infants and children: A review of its complex and synergistic interactions. Paediatr. Int. Child Health 2014, 34, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.H.; Rivera, J.A.; Bhutta, Z.; Gibson, R.S.; King, J.C.; Lonnerdal, B.; Ruel, M.T.; Sandtrom, B.; Wasantwisut, E.; Hotz, C. International zinc nutrition consultative group (IZINCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 2004, 25, S99–S203. [Google Scholar] [PubMed]
- Davies, I.J.; Musa, M.; Dormandy, T.L. Measurements of plasma zinc. I. In health and disease. J. Clin. Pathol. 1968, 21, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Bresnahan, K.A.; Tanumihardjo, S.A. Undernutrition, the acute phase response to infection, and its effects on micronutrient status indicators. Adv. Nutr. 2014, 5, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Dineley, K.E.; Malaiyandi, L.M.; Reynolds, I.J. A reevaluation of neuronal zinc measurements: Artifacts associated with high intracellular dye concentration. Mol. Pharmacol. 2002, 62, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [PubMed]
- Ding, W.Q.; Lind, S.E. Metal ionophores—An emerging class of anticancer drugs. IUBMB Life 2009, 61, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Watjen, W.; Beyersmann, D. Zinc induces apoptosis that can be suppressed by lanthanum in C6 rat glioma cells. Biol. Chem. 2001, 382, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Domaille, D.W.; Que, E.L.; Chang, C.J. Synthetic fluorescent sensors for studying the cell biology of metals. Nat. Chem. Biol. 2008, 4, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Tomat, E.; Lippard, S.J. Imaging mobile zinc in biology. Curr. Opin. Chem. Biol. 2010, 14, 225–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueroa, J.A.; Vignesh, K.S.; Deepe, G.S., Jr.; Caruso, J. Selectivity and specificity of small molecule fluorescent dyes/probes used for the detection of Zn2+ and Ca2+ in cells. Metallomics 2014, 6, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.L.; Stork, C.J.; Li, Y.V. Determining zinc with commonly used calcium and zinc fluorescent indicators, a question on calcium signals. Cell Calcium 2006, 40, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.P.; Blindauer, C.A.; Kassaar, O.; Khazaipoul, S.; Martin, E.M.; Sadler, P.J.; Stewart, A.J. Allosteric modulation of zinc speciation by fatty acids. Biochim. Biophys. Acta 2013, 1830, 5456–5464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krezel, A.; Maret, W. Dual nanomolar and picomolar Zn(II) binding properties of metallothionein. J. Am. Chem. Soc. 2007, 129, 10911–10921. [Google Scholar] [CrossRef] [PubMed]
- Kehl-Fie, T.E.; Chitayat, S.; Hood, M.I.; Damo, S.; Restrepo, N.; Garcia, C.; Munro, K.A.; Chazin, W.J.; Skaar, E.P. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of staphylococcus aureus. Cell Host Microbe 2011, 10, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Brophy, M.B.; Hayden, J.A.; Nolan, E.M. Calcium ion gradients modulate the zinc affinity and antibacterial activity of human calprotectin. J. Am. Chem. Soc. 2012, 134, 18089–18100. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.R. Thermodynamic binding constants of the zinc-human serum transferrin complex. Biochemistry 1983, 22, 3920–3926. [Google Scholar] [CrossRef] [PubMed]
- Burdette, S.C.; Walkup, G.K.; Spingler, B.; Tsien, R.Y.; Lippard, S.J. Fluorescent sensors for Zn2+ based on a fluorescein platform: Synthesis, properties and intracellular distribution. J. Am. Chem. Soc. 2001, 123, 7831–7841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gee, K.R.; Zhou, Z.L.; Ton-That, D.; Sensi, S.L.; Weiss, J.H. Measuring zinc in living cells. A new generation of sensitive and selective fluorescent probes. Cell Calcium 2002, 31, 245–251. [Google Scholar] [CrossRef]
- Fluorescent indicators for zinc. Available online: https://tools.Thermofisher.Com/content/sfs/manuals/mp07990.Pdf (accessed on 10 January 2016).
- Gee, K.R.; Zhou, Z.L.; Qian, W.J.; Kennedy, R. Detection and imaging of zinc secretion from pancreatic beta-cells using a new fluorescent zinc indicator. J. Am. Chem. Soc. 2002, 124, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Canzoniero, L.M.; Turetsky, D.M.; Choi, D.W. Measurement of intracellular free zinc concentrations accompanying zinc-induced neuronal death. J. Neurosci. 1999, 19, RC31. [Google Scholar] [PubMed]
- Hechtenberg, S.; Beyersmann, D. Differential control of free calcium and free zinc levels in isolated bovine liver nuclei. Biochem. J. 1993, 289, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Atar, D.; Backx, P.H.; Appel, M.M.; Gao, W.D.; Marban, E. Excitation-transcription coupling mediated by zinc influx through voltage-dependent calcium channels. J. Biol. Chem. 1995, 270, 2473–2477. [Google Scholar] [CrossRef] [PubMed]
- Simons, T.J. Measurement of free Zn2+ ion concentration with the fluorescent probe mag-fura-2 (furaptra). J. Biochem. Biophys. Methods 1993, 27, 25–37. [Google Scholar] [CrossRef]
- Sensi, S.L.; Canzoniero, L.M.; Yu, S.P.; Ying, H.S.; Koh, J.Y.; Kerchner, G.A.; Choi, D.W. Measurement of intracellular free zinc in living cortical neurons: Routes of entry. J. Neurosci. 1997, 17, 9554–9564. [Google Scholar] [PubMed]
- Zalewski, P.D.; Forbes, I.J.; Seamark, R.F.; Borlinghaus, R.; Betts, W.H.; Lincoln, S.F.; Ward, A.D. Flux of intracellular labile zinc during apoptosis (gene-directed cell death) revealed by a specific chemical probe, zinquin. Chem. Biol. 1994, 1, 153–161. [Google Scholar] [CrossRef]
- Meeusen, J.W.; Tomasiewicz, H.; Nowakowski, A.; Petering, D.H. Tsq (6-methoxy-8-p-toluenesulfonamido-quinoline), a common fluorescent sensor for cellular zinc, images zinc proteins. Inorg. Chem. 2011, 50, 7563–7573. [Google Scholar] [CrossRef] [PubMed]
- Aggett, P.J. Population reference intakes and micronutrient bioavailability: A european perspective. Am. J. Clin. Nutr. 2010, 91, 1433s–1437s. [Google Scholar] [CrossRef] [PubMed]
- Moran, V.H.; Stammers, A.L.; Medina, M.W.; Patel, S.; Dykes, F.; Souverein, O.W.; Dullemeijer, C.; Perez-Rodrigo, C.; Serra-Majem, L.; Nissensohn, M.; et al. The relationship between zinc intake and serum/plasma zinc concentration in children: A systematic review and dose-response meta-analysis. Nutrients 2012, 4, 841–858. [Google Scholar] [CrossRef] [PubMed]
- Sandstrom, B.; Cederblad, A. Zinc absorption from composite meals. II. Influence of the main protein source. Am. J. Clin. Nutr. 1980, 33, 1778–1783. [Google Scholar] [PubMed]
- Lonnerdal, B. Dietary factors influencing zinc absorption. J. Nutr. 2000, 130, 1378s–1383s. [Google Scholar] [PubMed]
- Gibson, R.S. A historical review of progress in the assessment of dietary zinc intake as an indicator of population zinc status. Adv. Nutr. 2012, 3, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.V.; Krebs, N.F.; Hambidge, K.M. A mathematical model of zinc absorption in humans as a function of dietary zinc and phytate. J. Nutr. 2007, 137, 135–141. [Google Scholar] [PubMed]
- Hambidge, K.M.; Miller, L.V.; Westcott, J.E.; Sheng, X.; Krebs, N.F. Zinc bioavailability and homeostasis. Am. J. Clin. Nutr. 2010, 91, 1478s–1483s. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.V.; Hambidge, K.M.; Krebs, N.F. Zinc absorption is not related to dietary phytate intake in infants and young children based on modeling combined data from multiple studies. J. Nutr. 2015, 145, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Hambidge, K.M.; Krebs, N.F.; Westcott, J.E.; Miller, L.V. Changes in zinc absorption during development. J. Pediatr. 2006, 149, S64–S68. [Google Scholar] [CrossRef] [PubMed]
- Jou, M.Y.; Philipps, A.F.; Kelleher, S.L.; Lonnerdal, B. Effects of zinc exposure on zinc transporter expression in human intestinal cells of varying maturity. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Overbeck, S.; Rink, L.; Haase, H. Modulating the immune response by oral zinc supplementation: A single approach for multiple diseases. Arch. Immunol. Ther. Exp. 2008, 56, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.B. pH as a variable in free zinc ion concentration from zinc-containing lozenges. Antimicrob. Agents Chemother. 1988, 32, 608–609. [Google Scholar] [CrossRef] [PubMed]
- Tucci, E.R.; Ke, C.H.; Li, N.C. Linear free energy relationships for proton dissociation and metal complexation of pyrimidine acids. J. Inorg. Nucl. Chem. 1967, 29, 1657. [Google Scholar] [CrossRef]
- Rosado, J.L. Zinc and copper: Proposed fortification levels and recommended zinc compounds. J. Nutr. 2003, 133, 2985s–2989s. [Google Scholar] [PubMed]
- Herman, S.; Griffin, I.J.; Suwarti, S.; Ernawati, F.; Permaesih, D.; Pambudi, D.; Abrams, S.A. Cofortification of iron-fortified flour with zinc sulfate, but not zinc oxide, decreases iron absorption in indonesian children. Am. J. Clin. Nutr. 2002, 76, 813–817. [Google Scholar] [PubMed]
- De Romana, D.L.; Lonnerdal, B.; Brown, K.H. Absorption of zinc from wheat products fortified with iron and either zinc sulfate or zinc oxide. Am. J. Clin. Nutr. 2003, 78, 279–283. [Google Scholar]
- Degerud, E.M.; Manger, M.S.; Strand, T.A.; Dierkes, J. Bioavailability of iron, vitamin A, zinc, and folic acid when added to condiments and seasonings. Ann. N. Y. Acad. Sci. 2015, 1357, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.M.; Brewer, G.J.; Dressman, J.B.; Swidan, S.Z.; DuRoss, D.J.; Adair, C.H.; Barnett, J.L.; Berardi, R.R. Effect of intragastric pH on the absorption of oral zinc acetate and zinc oxide in young healthy volunteers. J. Parenter. Enter. Nutr. 1995, 19, 393–397. [Google Scholar] [CrossRef]
- Valberg, L.S.; Flanagan, P.R.; Chamberlain, M.J. Effects of iron, tin, and copper on zinc absorption in humans. Am. J. Clin. Nutr. 1984, 40, 536–541. [Google Scholar] [PubMed]
- Solomons, N.W.; Jacob, R.A. Studies on the bioavailability of zinc in humans: Effects of heme and nonheme iron on the absorption of zinc. Am. J. Clin. Nutr. 1981, 34, 475–482. [Google Scholar] [PubMed]
- Whittaker, P. Iron and zinc interactions in humans. Am. J. Clin. Nutr. 1998, 68, 442s–446s. [Google Scholar] [PubMed]
- Lindenmayer, G.W.; Stoltzfus, R.J.; Prendergast, A.J. Interactions between zinc deficiency and environmental enteropathy in developing countries. Adv. Nutr. 2014, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]








| Zinc-Binding Protein | KD | Conditions | Reference |
|---|---|---|---|
| Human serum albumin | 30–100 nM | pH dependent | [48] |
| Metallothionein | ~1.6 nM, ~10 nM and ~0.2 µM for the different binding sites | pH 7.4 | [49] |
| Calprotectin S100A8, S100A9 | 1.35 nM for the first and 5.6 nM for the second zinc ion | in the absence of Ca2+ | [50] |
| ≤10 pM for the first and ≤240 pM for the second zinc ion | in the presence of Ca2+ | [51] | |
| Human serum transferrin | 16 nM and 0.4 µM | in 0.1 M N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES), 15 mM bicarbonate at pH 7.4 and 25 °C | [52] |
| Zn2+ Indicator | KD | Buffer System | Reference |
|---|---|---|---|
| Zinpyr-1 | 0.7 ± 0.1 nM | Ca2+/EDTA/Zn2+ buffer at pH 7.0 | [53] |
| Zinpyr-2 | 0.5 ± 0.1 nM | Ca2+/EDTA/Zn2+ buffer at pH 7.0 | [53] |
| FluoZin-1 | 7.8 µM | unbuffered Zn2+ solution at pH 7.0 | [54] |
| 8 µM | 50 mM MOPS at pH 7.0 and 22 °C | [55] | |
| Fluozin-2 | 2.1 µM | unbuffered Zn2+ solution at pH 7.0 | [54] |
| 2 µM | 50 mM MOPS at pH 7.0 and 22 °C | [55] | |
| FluoZin-3 | 8.9 nM | 1mM metal buffer containing 0.05–0.95 mM ZnCl2 in 50 mM HEPES at pH 7.4 and 25 °C, I = 0.1 M | [5] |
| 15 nM | 20 mM HEPES, 135 mM NaCl, 1.1 mM total EGTA, and 0−1.1 mM ZnCl2 at pH 7.4 and 22 °C | [56] | |
| 135 mM NaCl, 1.1 mM EGTA, 20 mM HEPES, 0–10 µM free Zn2+ at pH 7.4 and 22 °C | [55] | ||
| Newport Green DCF | 1 µM | EGTA-buffered Zn2+ | [57] |
| 50 mM 3-morpholinopropane-1-sulfonic acid (MOPS) pH 7.0 at 22 °C | [55] | ||
| Newport Green PDX | 40 µM | unbuffered Zn2+ solutions at pH 7.0 | [54] |
| 30 µM | 50 mM MOPS at pH 7.0 and 22 °C | [55] | |
| FuraZin | 3.4 µM | unbuffered Zn2+ solution at pH 7.0 | [54] |
| Fura-2 AM | 1.5 nM | 125 mM KCI, 2 mM K2HPO4, 25 mM HEPES, 4 mM MgCl2, 0.5 mM EGTA, 0.5 mM EDTA, 2 mM NTA at pH 7.0 and 25 °C | [58] |
| Fura-2 | 3 nM | 100 mM KCl, 10 mM HEPES, 10 mM EGTA, 20 mM NTA, 1 mM CaCl2, 10−3 mM fura-2 and varied amounts of ZnCl2 at pH 7.15 | [59] |
| Mag-fura-2 | 20 nM | at pH 7.0–7.8 and 37 °C | [60] |
| Mag-fura-5 | 27 nM | 100 mM KCl, 0.1 mM EGTA, 10 mM MOPS and 0–0.2 mM ZnCl2 at pH 7 and room temperature | [61] |
| RhodZin | 23.0 µM | unbuffered Zn2+ solution at pH 7.0 | [54] |
| RhodZin-3 | 65 nM | 50 mM MOPS at pH 7.0 and 22 °C | [55] |
| Zinquin | 370 ± 60 nM (1:1 complex) and 85 ± 16 nM (2:1 complex) | in physiological medium | [62] |
| TSQ | 15.5 µM | dissociation of Zn-carbonic anhydrase | [63] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ollig, J.; Kloubert, V.; Weßels, I.; Haase, H.; Rink, L. Parameters Influencing Zinc in Experimental Systems in Vivo and in Vitro. Metals 2016, 6, 71. https://doi.org/10.3390/met6030071
Ollig J, Kloubert V, Weßels I, Haase H, Rink L. Parameters Influencing Zinc in Experimental Systems in Vivo and in Vitro. Metals. 2016; 6(3):71. https://doi.org/10.3390/met6030071
Chicago/Turabian StyleOllig, Johanna, Veronika Kloubert, Inga Weßels, Hajo Haase, and Lothar Rink. 2016. "Parameters Influencing Zinc in Experimental Systems in Vivo and in Vitro" Metals 6, no. 3: 71. https://doi.org/10.3390/met6030071
APA StyleOllig, J., Kloubert, V., Weßels, I., Haase, H., & Rink, L. (2016). Parameters Influencing Zinc in Experimental Systems in Vivo and in Vitro. Metals, 6(3), 71. https://doi.org/10.3390/met6030071