An Insight into Evolution of Light Weight High Entropy Alloys: A Review
Abstract
:1. Introduction
2. Basic Principles of LWHEA Designing
2.1. Core Effects of HEA
2.2. Thermodynamic Parameters for the Formation of Solid Solution and Metallic Glass
2.3. Non-Equiatomic Composition as a Better Design Approach
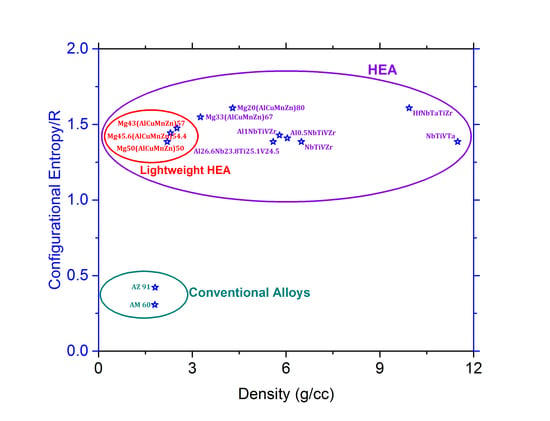
2.4. Theoretical Aspects and Designing of a LWHEA
3. Processing of LWHEAs
3.1. Melting and Casting Route
3.2. Mechanical Alloying and Consolidation
4. Microstructural Characteristics of LWHEA
5. Mechanical Properties of LWHEA
6. Future Directions for the Development LWHEAs
6.1. Synthesis of Porous Structured LWHEA
6.2. Use of Powder Metallurgy with Microwave Sintering Route
6.3. Synthesis of LWHEA Using Disintegration Meld Deposition Technique
6.4. Use of Additive Manufacturing Technique
7. Research Lapses
Conflicts of Interest
References
- Schubert, E.; Klassen, M.; Zerner, I.; Walz, C.; Sepold, G. Light-weight structures produced by laser beam joining for future applications in automobile and aerospace industry. J. Mater. Process. Technol. 2001, 115, 2–8. [Google Scholar] [CrossRef]
- Cheah, L.W. Cars on a diet: The Material and Energy Impacts of Passenger Vehicle Weight Reduction in the US. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2010. [Google Scholar]
- Miller, W.; Zhuang, L.; Bottema, J.; Wittebrood, A.J.; De Smet, P.; Haszler, A.; Vieregge, A. Recent development in aluminium alloys for the automotive industry. Mater. Sci. Eng. A 2000, 280, 37–49. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Lin, S.-J.; Chin, T.-S.; Gan, J.-Y.; Chen, S.-K.; Shun, T.-T.; Tsau, C.-H.; Chou, S.-Y. Formation of simple crystal structures in Cu-Co-Ni-Cr-Al-Fe-Ti-V alloys with multiprincipal metallic elements. Metall. Mater. Trans. A 2004, 35, 2533–2536. [Google Scholar] [CrossRef]
- Tong, C.-J.; Chen, Y.-L.; Yeh, J.-W.; Lin, S.-J.; Chen, S.-K.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Microstructure characterization of AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A 2005, 36, 881–893. [Google Scholar] [CrossRef]
- Chen, T.-K.; Shun, T.; Yeh, J.; Wong, M. Nanostructured nitride films of multi-element high-entropy alloys by reactive DC sputtering. Surf. Coat. Technol. 2004, 188, 193–200. [Google Scholar] [CrossRef]
- Tong, C.-J.; Chen, M.-R.; Yeh, J.-W.; Lin, S.-J.; Chen, S.-K.; Shun, T.-T.; Chang, S.-Y. Mechanical performance of the AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater.Trans. A 2005, 36, 1263–1271. [Google Scholar] [CrossRef]
- Huang, P.K.; Yeh, J.W.; Shun, T.T.; Chen, S.K. Multi-Principal-Element Alloys with Improved Oxidation and Wear Resistance for Thermal Spray Coating. Adv. Eng. Mater. 2004, 6, 74–78. [Google Scholar] [CrossRef]
- Chen, Y.; Duval, T.; Hung, U.; Yeh, J.; Shih, H. Microstructure and electrochemical properties of high entropy alloys—A comparison with type-304 stainless steel. Corros. Sci. 2005, 47, 2257–2279. [Google Scholar] [CrossRef]
- Chen, Y.; Hong, U.; Shih, H.; Yeh, J.; Duval, T. Electrochemical kinetics of the high entropy alloys in aqueous environments—A comparison with type 304 stainless steel. Corros. Sci. 2005, 47, 2679–2699. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Yeh, J.-W.; Chen, S.-K.; Shun, T.-T. Wear resistance and high-temperature compression strength of Fcc CuCoNiCrAl0.5Fe alloy with boron addition. Metall. Mater. Trans. A 2004, 35, 1465–1469. [Google Scholar] [CrossRef]
- Gao, M.C.; Yeh, J.-W.; Liaw, P.K.; Zhang, Y. High-Entropy Alloys: Fundamentals and Applications; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Industrial Development of High-Entropy Alloys. Masson. Available online: https://www.scribd.com/document/269966894/The-Industrial-Development-of-High-Entropy-Alloys (accessed on 24 August 2016).
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Glicksman, M.E. Principles of Solidification: An Introduction to Modern Casting and Crystal Growth Concepts; Springer Science & Business Media: New York, NY, USA, 2010. [Google Scholar]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Huang, Y.-S. Recent Patents on High-Entropy Alloy. Recent Pat. Mater. Sci. 2009, 2, 154–157. [Google Scholar] [CrossRef]
- Frazier, W.E.; Lee, E.W.; Donnellan, M.E.; Thompson, J.J. Advanced lightweight alloys for aerospace applications. JOM 1989, 41, 22–26. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Liaw, P. Microstructure and compressive properties of NbTiVTaAlx high entropy alloys. Proced. Eng. 2012, 36, 292–298. [Google Scholar] [CrossRef]
- Senkov, O.; Scott, J.; Senkova, S.; Miracle, D.; Woodward, C. Microstructure and room temperature properties of a high-entropy TaNbHfZrTi alloy. J. Alloy. Compd. 2011, 509, 6043–6048. [Google Scholar] [CrossRef]
- Lilensten, L.; Couzinié, J.; Perrière, L.; Bourgon, J.; Emery, N.; Guillot, I. New structure in refractory high-entropy alloys. Mater. Lett. 2014, 132, 123–125. [Google Scholar] [CrossRef]
- Senkov, O.; Senkova, S.; Woodward, C.; Miracle, D. Low-density, refractory multi-principal element alloys of the Cr-Nb-Ti-V-Zr system: Microstructure and phase analysis. Acta Mater. 2013, 61, 1545–1557. [Google Scholar] [CrossRef]
- Senkov, O.; Senkova, S.; Miracle, D.; Woodward, C. Mechanical properties of low-density, refractory multi-principal element alloys of the Cr-Nb-Ti-V-Zr system. Mater. Sci. Eng. A 2013, 565, 51–62. [Google Scholar] [CrossRef]
- Stepanov, N.; Yurchenko, N.Y.; Shaysultanov, D.; Salishchev, G.; Tikhonovsky, M. Effect of Al on structure and mechanical properties of AlxNbTiVZr (x = 0, 0.5, 1, 1.5) high entropy alloys. Mater. Sci. Technol. 2015, 31, 1184–1193. [Google Scholar] [CrossRef]
- A Novel Light High-Entropy Alloy Al20Be20Fe10Si15Ti35. Available online: http://www.science24.com/paper/19071 (accessed on 25 August 2016).
- Li, R.; Gao, J.C.; Fan, K. Study to microstructure and mechanical properties of Mg containing high entropy alloys. Mater. Sci. Forum 2010, 650, 265–271. [Google Scholar] [CrossRef]
- Li, R.; Gao, J.C.; Fan, K. Microstructure and mechanical properties of MgMnAlZnCu high entropy alloy cooling in three conditions. Mater. Sci. Forum 2011, 686, 235–241. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Tsai, C.-W.; Juan, C.-C.; Chuang, M.-H.; Yeh, J.-W.; Chin, T.-S.; Chen, S.-K. Amorphization of equimolar alloys with HCP elements during mechanical alloying. J. Alloy. Compd. 2010, 506, 210–215. [Google Scholar] [CrossRef]
- Youssef, K.M.; Zaddach, A.J.; Niu, C.; Irving, D.L.; Koch, C.C. A novel low-density, high-hardness, high-entropy alloy with close-packed single-phase nanocrystalline structures. Mater. Res. Lett. 2015, 3, 95–99. [Google Scholar] [CrossRef]
- Gaskell, D.R. Introduction to the Thermodynamics of Materials; CRC Press: Boca Raton, LA, USA, 2008. [Google Scholar]
- Jien-Wei, Y. Recent progress in high entropy alloys. Ann. Chim. Sci. Mat. 2006, 31, 633–648. [Google Scholar]
- Yeh, J.-W. Alloy design strategies and future trends in high-entropy alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Tsai, K.-Y.; Tsai, M.-H.; Yeh, J.-W. Sluggish diffusion in Co-Cr-Fe-Mn-Ni high-entropy alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Takeuchi, A.; Amiya, K.; Wada, T.; Yubuta, K.; Zhang, W.; Makino, A. Entropies in alloy design for high-entropy and bulk glassy alloys. Entropy 2013, 15, 3810–3821. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Liaw, P. Alloy design and properties optimization of high-entropy alloys. JOM 2012, 64, 830–838. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Sheng, G.; Liu, C.T. Phase stability in high entropy alloys: formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar]
- Varalakshmi, S.; Kamaraj, M.; Murty, B. Processing and properties of nanocrystalline CuNiCoZnAlTi high entropy alloys by mechanical alloying. Mater.Sci. Eng. A 2010, 527, 1027–1030. [Google Scholar] [CrossRef]
- Senkov, O.; Wilks, G.; Scott, J.; Miracle, D. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.; Knight, P.; Vincent, A. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Pradeep, K.; Wanderka, N.; Choi, P.; Banhart, J.; Murty, B.; Raabe, D. Atomic-scale compositional characterization of a nanocrystalline AlCrCuFeNiZn high-entropy alloy using atom probe tomography. Acta Mater. 2013, 61, 4696–4706. [Google Scholar] [CrossRef]
- Otto, F.; Yang, Y.; Bei, H.; George, E.P. Relative effects of enthalpy and entropy on the phase stability of equiatomic high-entropy alloys. Acta Mater. 2013, 61, 2628–2638. [Google Scholar] [CrossRef]
- Singh, S.; Wanderka, N.; Murty, B.; Glatzel, U.; Banhart, J. Decomposition in multi-component AlCoCrCuFeNi high-entropy alloy. Acta Mater. 2011, 59, 182–190. [Google Scholar] [CrossRef]
- Shun, T.-T.; Chang, L.-Y.; Shiu, M.-H. Microstructure and mechanical properties of multiprincipal component CoCrFeNiMox alloys. Mater. Charact. 2012, 70, 63–67. [Google Scholar] [CrossRef]
- Singh, S.; Wanderka, N.; Kiefer, K.; Siemensmeyer, K.; Banhart, J. Effect of decomposition of the Cr-Fe-Co rich phase of AlCoCrCuFeNi high entropy alloy on magnetic properties. Ultramicroscopy 2011, 111, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Tasan, C.C.; Pradeep, K.G.; Springer, H.; Kostka, A.; Raabe, D. Design of a twinning-induced plasticity high entropy alloy. Acta Mater. 2015, 94, 124–133. [Google Scholar] [CrossRef]
- Tasan, C.C.; Deng, Y.; Pradeep, K.G.; Yao, M.; Springer, H.; Raabe, D. Composition dependence of phase stability, deformation mechanisms, and mechanical properties of the CoCrFeMnNi high-entropy alloy system. JOM 2014, 66, 1993–2001. [Google Scholar] [CrossRef]
- Yao, M.; Pradeep, K.; Tasan, C.; Raabe, D. A novel, single phase, non-equiatomic FeMnNiCoCr high-entropy alloy with exceptional phase stability and tensile ductility. Scr. Mater. 2014, 72, 5–8. [Google Scholar] [CrossRef]
- Raabe, D.; Tasan, C.C.; Springer, H.; Bausch, M. From High-Entropy Alloys to High-Entropy Steels. Steel Res. Int. 2015, 86, 1127–1138. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, Q.; Lu, J.; Liu, C.; Yang, Y. Design of high entropy alloys: A single-parameter thermodynamic rule. Scr. Mater. 2015, 104, 53–55. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, Q.; Lu, J.; Liu, C.; Yang, Y. The generalized thermodynamic rule for phase selection in multicomponent alloys. Intermetallics 2015, 59, 75–80. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef]
- Gao, M.C.; Alman, D.E. Searching for next single-phase high-entropy alloy compositions. Entropy 2013, 15, 4504–4519. [Google Scholar] [CrossRef]
- Senkov, O.; Miller, J.; Miracle, D.; Woodward, C. Accelerated exploration of multi-principal element alloys with solid solution phases. Nat. Commun. 2015, 6, 6529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, F.; Chen, S.; Cao, W. Computational thermodynamics aided high-entropy alloy design. JOM 2012, 64, 839–845. [Google Scholar] [CrossRef]
- Miracle, D.B.; Miller, J.D.; Senkov, O.N.; Woodward, C.; Uchic, M.D.; Tiley, J. Exploration and development of high entropy alloys for structural applications. Entropy 2014, 16, 494–525. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, M.C. CALPHAD Modeling of High-Entropy Alloys, in High-Entropy Alloys; Springer: Cham, Switzerland, 2016; pp. 399–444. [Google Scholar]
- Ye, Y.; Wang, Q.; Lu, J.; Liu, C.; Yang, Y. High-entropy alloy: challenges and prospects. Mater. Today 2015, 19, 349–362. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, B.; Guo, S.; Qiao, J.; Hawk, J. High-entropy alloys in hexagonal close-packed structure. Metall. Mater. Trans. A 2016, 47, 3322–3332. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Zhao, M.; Jiang, Q. Effect of alloying elements on microstructure and properties of multiprincipal elements high-entropy alloys. J. Alloy. Compd. 2009, 475, 752–757. [Google Scholar] [CrossRef]
- Schuh, B.; Mendez-Martin, F.; Völker, B.; George, E.; Clemens, H.; Pippan, R.; Hohenwarter, A. Mechanical properties, microstructure and thermal stability of a nanocrystalline CoCrFeMnNi high-entropy alloy after severe plastic deformation. Acta Mater. 2015, 96, 258–268. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Qiao, Y.; Chen, G. Novel microstructure and properties of multicomponent CoCrCuFeNiTix alloys. Intermetallics 2007, 15, 357–362. [Google Scholar] [CrossRef]
- Zhang, K.B.; Fu, Z.Y.; Zhang, J.Y.; Wang, W.M.; Wang, H.; Wang, Y.C.; Zhang, Q.J. Characterization of nanocrystalline CoCrFeNiCuAl high-entropy alloy powder processed by mechanical alloying. Mater. Sci. Forum 2009, 620, 383–386. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Kim, T.; Chen, G. Microstructure characterizations and strengthening mechanism of multi-principal component AlCoCrFeNiTi0.5 solid solution alloy with excellent mechanical properties. Mater. Lett. 2008, 62, 2673–2676. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Wang, F.; Chen, G. Phase transformation induced by lattice distortion in multiprincipal component CoCrFeNiCuxAl1−x solid-solution alloys. Appl. Phys. Lett. 2008, 92, 241917. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Quantitative evaluation of critical cooling rate for metallic glasses. Mater. Sci. Eng. A 2001, 304, 446–451. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Miedema, A.; de Chatel, P.; de Boer, F. Cohesion in alloys—Fundamentals of a semi-empirical model. Physica B C 1980, 100, 1–28. [Google Scholar] [CrossRef]
- Hammond, V.H.; Atwater, M.A.; Darling, K.A.; Nguyen, H.Q.; Kecskes, L.J. Equal-channel angular extrusion of a low-density high-entropy alloy produced by high-energy cryogenic mechanical alloying. JOM 2014, 66, 2021–2029. [Google Scholar] [CrossRef]
- Benjamin, J.S. Dispersion strengthened superalloys by mechanical alloying. Metall. Trans. 1970, 1, 2943–2951. [Google Scholar]
- Stepanov, N.; Shaysultanov, D.; Salishchev, G.; Tikhonovsky, M. Structure and mechanical properties of a light-weight AlNbTiV high entropy alloy. Mater. Lett. 2015, 142, 153–155. [Google Scholar] [CrossRef]
- Zardiackas, L.D.; Parsell, D.E.; Dillon, L.D.; Mitchell, D.W.; Nunnery, L.A.; Poggie, R. Structure, metallurgy, and mechanical properties of a porous tantalum foam. J. Biomed. Mater. Res. 2001, 58, 180–187. [Google Scholar] [CrossRef]
- Lefebvre, L.-P.; Banhart, J.; Dunand, D. Porous metals and metallic foams: Current status and recent developments. Adv. Eng. Mater. 2008, 10, 775–787. [Google Scholar] [CrossRef] [Green Version]
- Čapek, J.; Vojtěch, D. Effect of sintering conditions on the microstructural and mechanical characteristics of porous magnesium materials prepared by powder metallurgy. Mater. Sci. Eng. C 2014, 35, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-H.; Jung, H.-D.; Kim, S.-W.; Lee, S.-M.; Kim, H.-E.; Estrin, Y.; Koh, Y.-H. Production and bio-corrosion resistance of porous magnesium with hydroxyapatite coating for biomedical applications. Mater. Lett. 2013, 108, 122–124. [Google Scholar] [CrossRef]
- Čapek, J.; Vojtěch, D. Properties of porous magnesium prepared by powder metallurgy. Mater. Sci. Eng. C 2013, 33, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Cay, H.; Xu, H.; Li, Q. Mechanical behavior of porous magnesium/alumina composites with high strength and low density. Mater. Sci. Eng. A 2013, 574, 137–142. [Google Scholar] [CrossRef]
- German, R.M. Powder Metallurgy Science; Metal Powder Industries Federation: Princeton, NJ, USA, 1984. [Google Scholar]
- Schaffer, J.P.; Saxena, A.; Antolovich, S.D.; Sanders, T.; Warner, S.B. The Science and Design of Engineering Materials; MacGraw-Hill Irwin: Chicago, IL, USA, 1995. [Google Scholar]
- Gupta, M.; Wong, W. Enhancing overall mechanical performance of metallic materials using two-directional microwave assisted rapid sintering. Scr. Mater. 2005, 52, 479–483. [Google Scholar] [CrossRef]
- Hashmi, S. Comprehensive Materials Processing; Elsevier: Burlington, MA, USA, 2014. [Google Scholar]
- Gupta, M.; Lai, M.; Soo, C. Processing-microstructure-mechanical properties of an Al-Cu/SiC metal matrix composite synthesized using disintegrated melt deposition technique. Mater. Res. Bull. 1995, 30, 1525–1534. [Google Scholar] [CrossRef]
- Subramanian, J.; Guan, K.C.; Kuma, J.; Gupta, M. Feasibility study on utilizing carbon dioxide during the processing of Mg-Al alloys. J. Mater. Process. Technol. 2011, 211, 1416–1422. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Jayalakshmi, S.; Gupta, M. Effect of addition of mutually soluble and insoluble metallic elements on the microstructure, tensile and compressive properties of pure magnesium. Mater. Sci. Eng. A 2011, 530, 149–160. [Google Scholar] [CrossRef]
- Shanthi, M.; Gupta, M.; Jarfors, A.; Tan, M. Synthesis, characterization and mechanical properties of nano alumina particulate reinforced magnesium based bulk metallic glass composites. Mater. Sci. Eng. A 2011, 528, 6045–6050. [Google Scholar] [CrossRef]
- Gupta, M.; Lai, M.; Saravanaranganathan, D. Synthesis, microstructure and properties characterization of disintegrated melt deposited Mg/SiC composites. J. Mater. Sci. 2000, 35, 2155–2165. [Google Scholar] [CrossRef]
- Hassan, S.; Gupta, M. Development of ductile magnesium composite materials using titanium as reinforcement. J. Alloy. Compd. 2002, 345, 246–251. [Google Scholar] [CrossRef]
- Ho, K.; Gupta, M.; Srivatsan, T.S. The mechanical behavior of magnesium alloy AZ91 reinforced with fine copper particulates. Mater. Sci. Eng. A 2004, 369, 302–308. [Google Scholar] [CrossRef]
- Nguyen, Q.; Gupta, M. Enhancing compressive response of AZ31B magnesium alloy using alumina nanoparticulates. Compos. Sci. Technol. 2008, 68, 2185–2192. [Google Scholar] [CrossRef]
- Paramsothy, M.; Hassan, S.; Srikanth, N.; Gupta, M. Enhancing tensile/compressive response of magnesium alloy AZ31 by integrating with Al2O3 nanoparticles. Mater. Sci. Eng. A 2009, 527, 162–168. [Google Scholar] [CrossRef]
- Meenashisundaram, G.K.; Gupta, M. Low volume fraction nano-titanium particulates for improving the mechanical response of pure magnesium. J. Alloy. Compd. 2014, 593, 176–183. [Google Scholar] [CrossRef]
- Meenashisundaram, G.K.; Seetharaman, S.; Gupta, M. Enhancing overall tensile and compressive response of pure Mg using nano-TiB2 particulates. Mater. Charact. 2014, 94, 178–188. [Google Scholar] [CrossRef]
- Ng, C.; Savalani, M.; Man, H.; Gibson, I. Layer manufacturing of magnesium and its alloy structures for future applications. Virtual Phys. Prototyp. 2010, 5, 13–19. [Google Scholar] [CrossRef]
- Savalani, M.; Hao, L.; Harris, R.A. Evaluation of CO2 and Nd: YAG lasers for the selective laser sintering of HAPEX®. J. Eng. Manuf. 2006, 220, 171–182. [Google Scholar] [CrossRef] [Green Version]



| Elements | Mole Fraction () | Atomic Radius (r) | Density (g/cc) | Two Elements | (kJ/mol) | Two Elements | (kJ/mol) |
|---|---|---|---|---|---|---|---|
| Mg | 0.35 | 160 | 1.74 | Mg, Li | −0.33 | Li, Ti | 34.854 |
| Li | 0.2 | 151 | 0.53 | Mg, Al | −1.662 | Li, Zn | −6.893 |
| Al | 0.35 | 143 | 2.7 | Mg, Ti | 13.542 | Al, Ti | −40.481 |
| Ti | 0.5 | 146 | 4.51 | Mg, Zn | −3.445 | Al, Zn | 0.637 |
| Zn | 0.5 | 142 | 7.14 | Li, Al | −3.384 | Ti, Zn | −22.324 |
| Alloy | Density (g/cc) | Process | Phase | Source |
|---|---|---|---|---|
| NbTiVTa | 11.49 | Arc melting | BCC | [19] |
| HfNbTaTiZr | 9.94 | Arc melting | BCC | [20] |
| Hf27.5Nb5Ta5Ti35Zr27.5 | 8.48 | Arc melting | orthorhombic | [21] |
| NbTiVZr | 6.49 | Arc melting | BCC | [25] |
| Al0.5NbTiVZr | 6.04 | BCC | ||
| AlNbTiVZr | 5.79 | BCC | ||
| Al1.5NbTiVZr | 5.55 | BCC | ||
| Al26.6Nb23.8Ti25.1V24.5 | 5.59 | Casting | BCC | [74] |
| Al20Be20Fe10Si15Ti35 | 3.91 | Casting | 3 phases | [26] |
| Mg20(AlCuMnZn)80 | 4.29 | Induction melting | HCP+ Al-Mn icosahedral Quasi-crystal | [27] |
| Mg33(AlCuMnZn)67 | 3.26 | |||
| Mg43(AlCuMnZn)57 | 2.51 | |||
| Mg45.6(AlCuMnZn)54.4 | 2.30 | |||
| Mg50(AlCuMnZn)50 | 2.20 | |||
| Al20Li20Mg10Sc20Ti30 | 2.67 | MA | FCC | [30] |
| Alloy | Density (g/cc) | Micro-Hardness (Hv) | |
|---|---|---|---|
| As Solidified | Homogenised | ||
| NbTiVTa | 11.49 | NA | |
| HfNbTaTiZr | 9.94 | 390±8 | |
| Hf27.5Nb5Ta5Ti35Zr27.5 | 8.48 | 245 | |
| NbTiVZr | 6.49 | 380 ± 10 | 460 ± 10 |
| Al0.5NbTiVZr | 6.04 | 470 ± 10 | 500 ± 20 |
| AlNbTiVZr | 5.79 | 540 ± 10 | 550 ± 20 |
| Al1.5NbTiVZr | 5.55 | 620 ± 20 | 630 ± 30 |
| Al26.6Nb23.8Ti25.1V24.5 | 5.59 | 448 ± 12 | |
| Al20Be20Fe10Si15Ti35 | 3.91 | 911 | |
| Mg20(AlCuMnZn)80 | 4.29 | 429 | |
| Mg33(AlCuMnZn)67 | 3.26 | 315 | |
| Mg43(AlCuMnZn)57 | 2.51 | 255 | |
| Mg45.6(AlCuMnZn)54.4 | 2.30 | 225 | |
| Mg50(AlCuMnZn)50 | 2.20 | 178 | |
| Al20Li20Mg10Sc20Ti30 | 2.67 | 591.4 (as milled) | 499.6 (annealed at 500 °C) |
| Alloy | Density (g/cc) | Yield Stress (MPa) | Peak Stress (MPa) | Fracture Strain (%) |
|---|---|---|---|---|
| NbTiVTa | 11.49 | 1092 | NA | >50 |
| HfNbTaTiZr | 9.94 | 929 | NA | >50 |
| Hf27.5Nb5Ta5Ti35Zr27.5 | 8.48 | NA | NA | NA |
| NbTiVZr | 6.49 | 1320 | 1470 | 4.2 |
| Al0.5NbTiVZr | 6.04 | 960 | 1100 | 4 |
| AlNbTiVZr | 5.79 | 1080 | 1210 | 2.3 |
| Al1.5NbTiVZr | 5.55 | NA | 1310 | 0 |
| Al26.6Nb23.8Ti25.1V24.5 | 5.59 | 1020 | 1318 | 5 |
| Mg20(AlCuMnZn)80 | 4.29 | 428 | 428 | 3.29 |
| Mg33(AlCuMnZn)67 | 3.26 | 437 | 437 | 3.41 |
| Mg43(AlCuMnZn)57 | 2.51 | 500 | 500 | 3.72 |
| Mg45.6(AlCuMnZn)54.4 | 2.30 | 482 | 482 | 4.06 |
| Mg50(AlCuMnZn)50 | 2.20 | 340 | 400 | 4.83 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Gupta, M. An Insight into Evolution of Light Weight High Entropy Alloys: A Review. Metals 2016, 6, 199. https://doi.org/10.3390/met6090199
Kumar A, Gupta M. An Insight into Evolution of Light Weight High Entropy Alloys: A Review. Metals. 2016; 6(9):199. https://doi.org/10.3390/met6090199
Chicago/Turabian StyleKumar, Amit, and Manoj Gupta. 2016. "An Insight into Evolution of Light Weight High Entropy Alloys: A Review" Metals 6, no. 9: 199. https://doi.org/10.3390/met6090199
APA StyleKumar, A., & Gupta, M. (2016). An Insight into Evolution of Light Weight High Entropy Alloys: A Review. Metals, 6(9), 199. https://doi.org/10.3390/met6090199