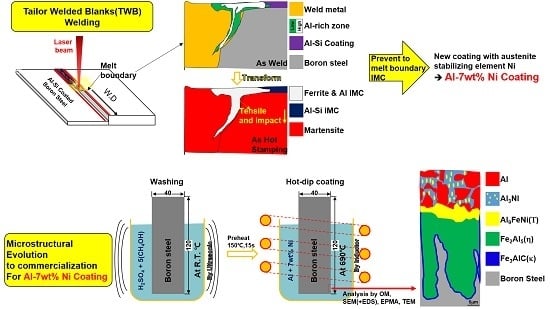
Microstructural Evolution of Intermetallic Compound Formed in Boron Steel Hot-Dipped in Al–7%Ni Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Microstructure of Coated Specimens
3.2. Microstructure of the Coating
3.3. Microstructure of the Reaction Layer
4. Discussion
5. Conclusions
- The cross-sectional microstructure formed by hot dipping was classified into a solidification microstructure of Al–7Ni alloy and a reaction layer. The reaction layer consisted of one layer on the surface of the steel and two layers inside the steel.
- The reaction phase formed on the original surface of the steel was the Al9FeNi (T) phase, which is a monoclinic (space group: P21/c) crystal structure. From a portion of the quasi-phase diagram of Al–7Ni–xFe (wt %) drawn using Thermo-Calc™, it was confirmed that this phase was formed if 2–5 wt % of the iron content in the Al–7Ni melt at 690 °C was dissolved.
- The reaction phase formed from the Al9FeNi (T) phase into the steel was the Fe2Al5 (η) phase, which is orthorhombic (space group: Cmcm). The variation in thickness of this phase increased linearly with increasing dipping time. This result is in accordance with diffusion growth, which is a growth mechanism of the Fe2Al5 (η) phase in Al-coated pure Fe and low-carbon steels. It is obvious that the thickness variation increased parabolically with time.
- The Fe3AlC (κ) phase, which had a band shape with a width of 100 nm, was formed at the interface of the Fe2Al5 (η) phase and the steel substrate. This phase had the same cubic crystal structure as Fe3Al. In addition, carbon was not detected in the Fe2Al5 (η) phase. Based on these results, it is considered that the Fe3AlC (κ) phase was formed by the diffusion of Al and C in the Fe2Al5 (η) phase toward the steel.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Åkerström, P. Modeling and Simulation of Hot Stamping. Ph.D. Thesis, Luleå University of Technology, Luleå, Sweden, 2006. [Google Scholar]
- Senuma, T. Physical metallurgy of modern high strength steel sheets. ISIJ Int. 2001, 41, 520–532. [Google Scholar] [CrossRef]
- Vaissiere, L.; Laurent, J.P.; Reinhardt, A. Development of pre-coated boron steel for applications on PSA Peugeot Citroen and Renault bodies in white. SAE Int. 2002. [Google Scholar] [CrossRef]
- Tetsuya, M.; Kohei, H.; Hidetaka, K. Ultra high-strength steel sheets for bodies, reinforcement parts, and seat frame parts of automobile—Ultra high-strength steel sheets leading to great improvement in crash-worthiness. JFE Tech. Rep. 2004, 4, 38–43. [Google Scholar]
- Aranda, L.; Garcia; Ravier, P.; Chastel, Y. Hot stamping of quenchable steels: Material data and process simulations. In Proceedings of the IDDRG 2003 Conference, Bled, Slovenia, 11–15 May 2003; pp. 155–164. [Google Scholar]
- Kolleck, R.; Veit, R.; Merklein, M.; Lechler, J.; Geiger, M. Investigation on induction heating for hot stamping of boron alloyed steels. CIRP Ann. Manuf. Technol. 2009, 58, 275–278. [Google Scholar] [CrossRef]
- Liu, H.S.; Xing, Z.W.; Bao, J.; Song, B.Y. Investigation of the hot-stamping process for advanced high-strength steel sheet by numerical simulation. J. Mater. Eng. Perform. 2010, 19, 325–334. [Google Scholar] [CrossRef]
- Manfred, G.; Marion, M.; Cornelia, H. Basic investigations on the hot stamping steel 22MnB5. Adv. Mater. Res. 2005, 6–8, 795–804. [Google Scholar]
- Neugebauera, R.; Altanb, T.; Geigerc, M.; Kleinerd, M.; Sterzinga, A. Sheet metal forming at elevated temperatures. CIRP Ann. Manuf. Technol. 2006, 55, 793–816. [Google Scholar] [CrossRef]
- Bahadur, A.; Mohanty, O.N. Aluminium diffusion coatings on medium carbon steel. Mater. Trans. JIM 1995, 36, 1170–1175. [Google Scholar] [CrossRef]
- Kobayashi, S.; Yakou, T. Control of intermetallic compound layers at interface between steel and aluminum by diffusion-treatment. Mater. Sci. Eng. A 2002, 338, 44–53. [Google Scholar] [CrossRef]
- Naoi, D.; Kajihara, M. Growth behavior of Fe2Al5 during reactive diffusion between Fe and Al at solid-state temperatures. Mater. Sci. Eng. A 2007, 459, 375–382. [Google Scholar] [CrossRef]
- Richards, R.W.; Jones, R.D.; Clements, P.D.; Clarke, H. Metallurgy of continuous hot dip aluminizing. Int. Mater. Rev. 1994, 39, 191–212. [Google Scholar] [CrossRef]
- Borsetto, F.; Ghiotti, A.; Bruschi, S. Investigation of the high strength steel Al-Si coating during hot stamping operations. Key Eng. Mater. 2009, 410–411, 289–296. [Google Scholar] [CrossRef]
- Karbasian, H.; Tekkaya, A.E. A review on hot stamping. J. Mater. Process. Technol. 2010, 210, 2103–2118. [Google Scholar] [CrossRef]
- Saunders, F.I.; Wagoner, R.H. Forming of tailor-welded blanks. Metall. Mater. Trans. A 1996, 27, 2605–2616. [Google Scholar] [CrossRef]
- Cretteur, L.; Vierstraete, R.; Yin, Q.; Ehling, W.; Pic, A. Development of a laser decoating process for fully functional Al Si coated press hardened steel laser welded blank solutions. In Proceedings of the 5th International WLT-Conference: Lasers in Manufacturing, Munich, Germany, 15–18 June 2009; pp. 409–413. [Google Scholar]
- Jung, B.H.; Kong, J.P.; Kang, C.Y. Effect of Hot-stamping Heat Treatment on the Microstructure of Al-Segregated Zone in TWB Laser Joints of Al-Si-coated Boron Steel and Zn-coated DP Steel. Korean J. Met. Mater. 2012, 50, 455–462. [Google Scholar]
- OH, M.-H.; Kong, J.-P.; Kwon, M.-S.; Kang, C.-Y. Effect of Hot-stamping on Microstructures and Tensile Properties of Al-Si Coated Boron Steel Welds with Laser Source. J. Weld. Join. 2013, 31, 96–106. [Google Scholar] [CrossRef]
- Yoon, T.-J.; Oh, M.-H.; Shin, H.-J.; Kang, C.-Y. Comparison of microstructure and phase transformation of laser-welded joints in Al-10wt% Si-coated boron steel before and after hot stamping. Mater. Charact. 2017, 128, 195–202. [Google Scholar] [CrossRef]
- Li, Y.S.; Spiegel, M. Models describing the degradation of FeAl and NiAl alloys induced by ZnCl 2–KCl melt at 400–450 °C. Corros. Sci. 2004, 46, 2009–2023. [Google Scholar] [CrossRef]
- Takata, N.; Nishimoto, M.; Kobayashi, S.; Takeyama, M. Morphology and formation of Fe-Al intermetallic layers on iron hot-dipped in Al-Mg-Si alloy melt. Intermetallics 2014, 54, 136–142. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Liao, Y.-J.; Wang, C.-J. Effect of nickel pre-plating on high-temperature oxidation behavior of hot-dipped aluminide mild steel. Mater. Charact. 2013, 82, 58–65. [Google Scholar] [CrossRef]
- Nash, P. Phase Diagrams of Binary Nickel Alloys; ASM International: Novelty, OH, USA, 1991; pp. 390–394. [Google Scholar]
- Wołczyński, W.; Kucharska, B.; Garzel, G.; Sypien, A.; Pogoda, Z.; Okane, T. Part III. Kinetics of the (Zn)-coating deposition during stable and meta-stable solidifications. Arch. Metall. Mater. 2015, 60, 199–207. [Google Scholar]
- Takata, N.; Nishimoto, M.; Kobayashi, S.; Takeyama, M. Crystallography of Fe2Al5 phase at the interface between solid Fe and liquid Al. Intermetallics 2015, 67, 1–11. [Google Scholar] [CrossRef]
- Springer, H.; Kostka, A.; Payton, E.J.; Raabe, D.; Kaysser-Pyzalla, A.; Eggeler, G. On the formation and growth of intermetallic phases during interdiffusion between low-carbon steel and aluminum alloys. Acta Mater. 2011, 59, 1586–1600. [Google Scholar] [CrossRef]
- Massalski, T.B.; Hiroaki, O. Binary Alloy Phase Diagrams; ASM International: Novelty, OH, USA, 1986. [Google Scholar]
- Schneider, A.; Zhang, J. Metal dusting of ferritic Fe-Al-M-C (M= Ti, V, Nb, Ta) alloys in CO-H2-H2O gas mixtures at 650 °C. Mater. Corros. 2003, 54, 778–784. [Google Scholar] [CrossRef]
- Schneider, A.; Zhang, J. Orientation relationship between a ferritic matrix and κ-phase (Fe3AlCx) precipitates formed during metal dusting of Fe-15Al. Intermetallics 2005, 13, 1332–1336. [Google Scholar] [CrossRef]
- Connétable, D.; Maugis, P. First principle calculations of the κ-Fe3AlC perovskite and iron-aluminium intermetallics. Intermetallics 2008, 16, 345–352. [Google Scholar] [CrossRef]












| No. | Chemical Composition (at. %) | Expected Phases | ||||
|---|---|---|---|---|---|---|
| Al | Ni | Si | Fe | Mn | ||
| 1 | 97.84 | 0.69 | 0.60 | 0.38 | 0.49 | Al |
| 2 | 83.36 | 14.50 | 0.80 | 0.86 | 0.48 | Al3Ni |
| 3 | 86.83 | 11.27 | 0.72 | 0.81 | 0.37 | Al + Al3Ni |
| 4 | 78.33 | 12.18 | 0.78 | 7.96 | 0.76 | Al9FeNi |
| 5* | 75.00 | 1.08 | 0.90 | 22.09 | 0.93 | Fe2Al5 |
| 5 | 71.41 | 0.88 | 1.08 | 25.44 | 1.19 | Fe2Al5 |
| No. | Al (at. %) | Fe (at. %) | Ni (at. %) | Expected Phases |
|---|---|---|---|---|
| 1 | 100 | - | - | Al |
| 2 | 75.74 | 2.08 | 22.18 | Al9FeNi |
| 3 | 76.03 | 8.23 | 15.74 | Al9FeNi |
| 4 | 76.85 | 10.72 | 12.42 | Al9FeNi |
| 5 | 71.67 | 28.33 | - | Fe2Al5 |
| 6 | 67.34 | 32.65 | - | Fe2Al5 |
| No. | Al (at. %) | Fe (at. %) | Mn (at. %) | C (at. %) | Expected Phases |
|---|---|---|---|---|---|
| 1 | 68.53 | 31.46 | - | - | Fe2Al5 |
| 2 | 18.51 | 65.31 | - | 16.17 | Fe3AlC |
| 3 | 0.20 | 97.80 | - | 0.32 | αFe |
| 4 | 76.85 | 78.23 | 4.24 | 17.52 | (Fe,Mn)3C |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, J.-G.; Lee, J.-H.; Kwak, S.-Y.; Kang, C.-Y. Microstructural Evolution of Intermetallic Compound Formed in Boron Steel Hot-Dipped in Al–7%Ni Alloy. Metals 2017, 7, 393. https://doi.org/10.3390/met7100393
Yun J-G, Lee J-H, Kwak S-Y, Kang C-Y. Microstructural Evolution of Intermetallic Compound Formed in Boron Steel Hot-Dipped in Al–7%Ni Alloy. Metals. 2017; 7(10):393. https://doi.org/10.3390/met7100393
Chicago/Turabian StyleYun, Jung-Gil, Jae-Hyeong Lee, Sung-Yun Kwak, and Chung-Yun Kang. 2017. "Microstructural Evolution of Intermetallic Compound Formed in Boron Steel Hot-Dipped in Al–7%Ni Alloy" Metals 7, no. 10: 393. https://doi.org/10.3390/met7100393
APA StyleYun, J. -G., Lee, J. -H., Kwak, S. -Y., & Kang, C. -Y. (2017). Microstructural Evolution of Intermetallic Compound Formed in Boron Steel Hot-Dipped in Al–7%Ni Alloy. Metals, 7(10), 393. https://doi.org/10.3390/met7100393