Mechanical, In Vitro Corrosion Resistance and Biological Compatibility of Cast and Annealed Ti25Nb10Zr Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Alloy
2.2. Composition and Structure of the Alloy
2.3. Mechanical Properties of the Alloy
2.4. In Vitro Corrosion Resistance of the Alloy
2.5. Wettability of the Alloy
2.6. In Vitro Biological Properties of the Alloy
3. Results and Discussion
3.1. Microchemical and Microstructural Properties
3.2. Mechanical Properties
3.3. Tribological Performance
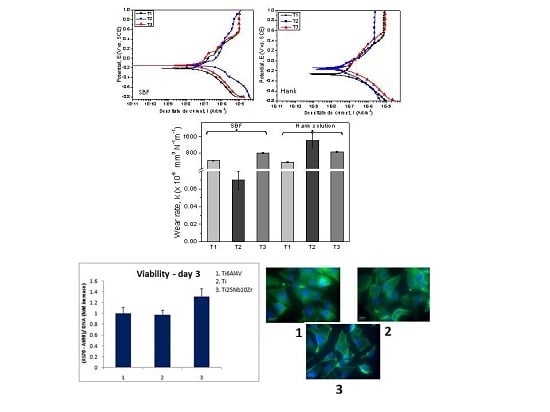
3.4. In Vitro Corrosion Resistance
3.5. Wettability
3.6. In Vitro Biological Properties
4. Discussions
5. Conclusions
- The cast Ti25Nb10Zr alloy is characterized by the presence of α + β phases. After annealing, enlarged dark grains appear delimited by a light network. Inside the delimited area, an acicular microstructure assigned to martensite with the needles oriented in the same direction was observed.
- The average hardness of the cast alloy was 2.3 GPa. After thermal treatment, the hardness increased by 1.8 GPa.
- The investigated alloy showed a low elastic modulus, 57.4 GPa (as-cast) and 52.3 GPa (annealed), being lower as compared to Ti6Al4V alloy (110 GPa).
- The yield strength of Ti25Nb10Zr alloy ranged from 427.2 MPa (as-cast) to 488.6 MPa (annealed), being lower than of Ti6Al4V alloy (930 MPa).
- The cast Ti25Nb10Zr alloy exhibits a lower friction coefficient in SBF (0.37) than that of annealed Ti25Nb10Zr alloy (0.70).
- The annealed Ti25Nb10Zr alloy has similar values of friction coefficient in both SBF and Hank solutions (µ ≈ 0.70).
- In SBF, a low wear rate was found for cast Ti25Nb10Zr alloy (0.07 × 10−6 mm3·N−1·m−1), followed by Ti6Al4V (413 × 10−6 mm3·N−1·m−1) and annealed Ti25Nb10Zr alloy (797 × 10−6 mm3·N−1·m−1).
- In Hank solution, the wear rate for cast and annealed Ti25Nb10Zr alloy ranged between 858 ÷ 1418 × 10−6 mm3·N−1·m−1 values, being higher than the values obtained in SBF solution.
- Independent of the electrolyte, both cast and annealed Ti25Nb10Zr alloys showed values of open circuit potential and corrosion potential that were more electropositive than the Ti6Al4V alloy. The low current density values were found in SBF for annealed (68.07 nA/cm2) and in Hank for cast 46.25 nA/cm2) alloy. The highest value of polarization resistance was measured for cast alloy tested in Hank solution. A material is resistant to corrosion when exhibiting electropositive values of Ecorr, high Rp and low icorr values. Taking into account these criteria, it can be seen that Ti25Nb10Zr alloy is more resistant to corrosion in SBF and Hank solutions when compared to the Ti6Al4V alloy, independent of their metallurgical conditions (cast or annealed).
- Cell viability and proliferation assay after five days showed that the Ti25Nb10Zr alloy exhibited good viability and proliferation with values of approximately 10% higher, respectively, than the ones registered for pure Ti.
- Osteocalcin and osteonectin gene expression of MG63 cells grown after three days on Ti25Nb10Zr surface is comparable to that recorded for pure Ti and higher than of Ti6Al4V alloy. After five days of culture, the osteocalcin expression level of Ti25Nb10Zr alloy is comparable to that recorded for the pure Ti and Ti6Al4V alloy, while the osteonectin expression level is slightly higher than both of them.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- United Nations Department of Economic; Social Affairs Population Division. World Population Ageing: 1950–2050; United Nations: New York, NY, USA, 2001.
- Itasca, I. National Safety Council—Injury Fact; National Safety Council: Itasca, IL, USA, 2015. [Google Scholar]
- Özcan, M.; Hämmerle, C. Titanium as a reconstruction and implant material in dentistry: Advantages and pitfalls. Materials (Basel) 2012, 5, 1528–1545. [Google Scholar] [CrossRef] [Green Version]
- Niinomi, M. Recent research and development in titanium alloys for biomedical applications and healthcare goods. Sci. Technol. Adv. Mater. 2003, 4, 445–454. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- De Almeida, L.H.; Bastos, I.N.; Santos, I.D.; Dutra, A.J. B.; Nunes, C.A.; Gabriel, S.B. Corrosion resistance of aged Ti-Mo-Nb alloys for biomedical applications. J. Alloy. Compd. 2015, 615, S666–S669. [Google Scholar] [CrossRef]
- Weinstein, A.; Horowitz, E.; Ruff, A.W. Retrieval and Analysis of Orthopaedic Implants; US Government printing office: Washingtin, DC, USA, 1977; pp. 23–30.
- BCC Research. Advanced Orthopedic Technologies, Implants and Regenerative Products; BCC Research: Wellesley, MA, USA, 2011. [Google Scholar]
- Biomaterials Market Overview. Avaliable online: https://www.alliedmarketresearch.com/biomaterials-market (accessed on 25 November 2016).
- Azevedo, C.R.F.; Hippert, E. Failure analysis of surgical implants in Brazil. Eng. Fail. Anal. 2002, 9, 621–633. [Google Scholar] [CrossRef]
- Miller, P.D.; Holladay, J.W. Friction and wear properties of titanium. Wear 1958, 2, 133–140. [Google Scholar] [CrossRef]
- Qu, J.; Blau, P.J.; Watkins, T.R.; Cavin, O.B.; Kulkarni, N.S. Friction and wear of titanium alloys sliding against metal, polymer, and ceramic counterfaces. Wear 2005, 258, 1348–1356. [Google Scholar] [CrossRef]
- Mao, C.; Li, H.; Cui, F.; Ma, C.; Feng, Q. Oriented growth of phosphates on polycrystalline titanium in a process mimicking biomineralization. J. Cryst. Growth 1999, 206, 308–321. [Google Scholar] [CrossRef]
- Mao, C.; Li, H.; Cui, F.; Feng, Q.; Ma, C. The functionalization of titanium with EDTA to induce biomimetic mineralization of hydroxyapatite. J. Mater. Chem. 1999, 9, 2573–2582. [Google Scholar] [CrossRef]
- Ning, C.; Yu, P.; Zhu, Y.; Yao, M.; Zhu, X.; Wang, X.; Lin, Z.; Li, W.; Wang, S.; Tan, G.; et al. Built-in microscale electrostatic fields induced by anatase–rutile-phase transition in selective areas promote osteogenesis. NPG Asia Mater. 2016, 8, e243. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Wang, S.; Zhu, Y.; Zhong, M.; Lin, X.; Zhang, Y.; Tan, G.; Li, M.; Yin, Z.; Yu, P.; et al. Ti nanorod arrays with a medium density significantly promote osteogenesis and osteointegration. Sci. Rep. 2016, 6, 19047. [Google Scholar] [CrossRef] [PubMed]
- Steinemann, S.G. Corrosion of surgical implants—In vivo and in vitro tests. In Evaluation of Biomaterials; Winter, D., Leray, L.J., de Groot, K., Eds.; John Wiley & Sons: New York, NY, USA, 1980; pp. 1–34. [Google Scholar]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Braic, M.; Vladescu, A.; Braic, V.; Cotrut, C.M.; Stanciu, D. Corrosion behaviour of Ti-10Nb-10Zr-5Ta alloys in artificial saliva solution with fluoride content. Mater. Corros. 2015, 66, 1331–1337. [Google Scholar] [CrossRef]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Guo, Z.; Jiang, Y.; Tao, X.; Liu, L. Study of low-modulus biomedical β Ti-Nb-Zr alloys based on single-crystal elastic constants modeling. J. Mech. Behav. Biomed. Mater. 2016, 62, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Ju, C.P.; Chern Lin, J.H. Structure-property relationship of cast Ti-Nb alloys. J. Oral Rehabil. 2002, 29, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Correa, D.R.N.; Vicente, F.B.; Donato, T.A.G.; Arana-Chavez, V.E.; Buzalaf, M.A.R.; Grandini, C.R. The effect of the solute on the structure, selected mechanical properties, and biocompatibility of Ti-Zr system alloys for dental applications. Mater. Sci. Eng. C 2014, 34, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.; Doi, H.; Yoneyama, T.; Hamanaka, H.; Gibson, I.R.; Best, S.M.; Shelton, J.C.; Bonfield, W. Influence of aging heat treatment on mechanical properties of biomedical Ti-Zr based ternary alloys containing niobium. J. Mater. Sci. Mater. Med. 1998, 9, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, T. Phase transformation and mechanical properties of the Ti50Zr30Nb10Ta10 alloy with low modulus and biocompatible. J. Alloy. Compd. 2005, 392, 291–294. [Google Scholar] [CrossRef]
- Cvijovic-Alagic, I.; Cvijovic, Z.; Mitrovic, S.; Panic, V.; Rakin, M. Wear and corrosion behaviour of Ti-13Nb-13Zr and Ti-6Al-4V alloys in simulated physiological solution. Corros. Sci. 2011, 53, 796–808. [Google Scholar] [CrossRef]
- Huang, H.H.; Wu, C.P.; Sun, Y.S.; Yang, W.E.; Lin, M.C.; Lee, T.H. Surface nanoporosity of β-type Ti-25Nb-25Zr alloy for the enhancement of protein adsorption and cell response. Surf. Coat. Technol. 2014, 259, 206–212. [Google Scholar] [CrossRef]
- BS-EN1071-13:2010 Advanced Technical Ceramics. Methods of Test for Ceramic Coatings. Determination of Wear Rate by the Pin-on-Disk Method. Available online: http://shop.bsigroup.com/ProductDetail/?pid=000000000030170505 (accessed on 6 March 2017).
- ASTM G102-89 (2004), Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. Available online: www.astm.org/database.cart/historical/g102-89r04e1 (accessed on 6 March 2017).
- Pinzari, F.; Ascarelli, P.; Cappelli, E.; Mattei, G.; Giorgi, R. Wettability of HF-CVD diamond films. Diam. Relat. Mater. 2001, 10, 781–785. [Google Scholar] [CrossRef]
- Fowkes, F.M. Additivity of intermolecular forces at interfaces. I. Determination of the contribution to surface and interfacial tensions of dispersion forces in various liquids. J. Phys. Chem. 1963, 67, 2538–2541. [Google Scholar] [CrossRef]
- Zenkiewicz, M. Methods for the calculation of surface free energy of solids. J. Achiev. Mater. Manuf. Eng. 2007, 24, 137–145. [Google Scholar]
- Zhao, X.; Niinomi, M.; Nakai, M.; Miyamoto, G.; Furuhara, T. Microstructures and mechanical properties of metastable Ti-30Zr-(Cr, Mo) alloys with changeable Young’s modulus for spinal fixation applications. Acta Biomater. 2011, 7, 3230–3236. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Jia, M.T.; Prima, F.; Hao, Y.L.; Yang, R. Improvements in nonlinear elasticity and strength by grain refinement in a titanium alloy with high oxygen content. Scr. Mater. 2011, 64, 1015–1018. [Google Scholar] [CrossRef]
- Guo, S.; Meng, Q.; Cheng, X.; Zhao, X. Microstructural evolution and mechanical behavior of metastable β-type Ti-30Nb-1Mo-4Sn alloy with low modulus and high strength. Prog. Nat. Sci. Mater. Int. 2015, 25, 414–418. [Google Scholar] [CrossRef]
- Guo, S.; Chen, B.; Meng, Q.; Zhao, R.; Zhao, X. Peculiar aging response of near β Ti-25Nb-2Mo-4Sn alloy for biomedical applications. Prog. Nat. Sci. Mater. Int. 2013, 23, 1–6. [Google Scholar] [CrossRef]
- Liu, Q.; Meng, Q.; Guo, S.; Zhao, X. α′ Type Ti-Nb-Zr alloys with ultra-low Young’s modulus and high strength. Prog. Nat. Sci. Mater. Int. 2013, 23, 562–565. [Google Scholar] [CrossRef]
- Meng, Q.; Guo, S.; Liu, Q.; Hu, L.; Zhao, X. A β-type TiNbZr alloy with low modulus and high strength for biomedical applications. Prog. Nat. Sci. Mater. Int. 2014, 24, 157–162. [Google Scholar] [CrossRef]
- Lai, M.; Gao, Y.; Yuan, B.; Zhu, M. Indirect determination of martensitic transformation temperature of sintered nickel-free Ti-22Nb-6Zr alloy by low temperature compression test. Mater. Des. 2014, 60, 193–197. [Google Scholar] [CrossRef]
- Anbarasan, V.; Jeya Ganesh, B.; Raju, S.; Murugesan, S.; Mohandas, E.; Kamachi Mudali, U.; Manivasagam, G. Thermal property characterization of a Ti-4 wt. %Nb-4 wt. %Zr alloy using drop and differential scanning calorimetry. J. Alloy. Compd. 2008, 463, 160–167. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Vladescu, A.; Braic, V.; Balaceanu, M.; Braic, M.; Parau, A.C.; Ivanescu, S.; Fanara, C. Characterization of the Ti-10Nb-10Zr-5Ta alloy for biomedical applications. Part 1: Microstructure, mechanical properties, and corrosion resistance. J. Mater. Eng. Perform. 2013, 22, 2389–2397. [Google Scholar] [CrossRef]
- Cottrell, A.H.; Artman, R.A. Mechanical Properties of Matter. Am. J. Phys. 1968, 36, 68–69. [Google Scholar] [CrossRef]
- Fan, Z.; Tsakiropoulos, P.; Smith, P.A.; Miodownik, A.P. Extension of the Hall-Petch relation to two-ductile-phase alloys. Philos. Mag. A 1993, 67, 515–531. [Google Scholar] [CrossRef]
- Galindo-Nava, E.I.; Rae, C.M.F. Microstructure-sensitive modelling of dislocation creep in polycrystalline FCC alloys: Orowan theory revisited. Mater. Sci. Eng. A 2016, 651, 116–126. [Google Scholar] [CrossRef]
- Scattergood, R.O.; Koch, C.C.; Murty, K.L.; Brenner, D. Strengthening mechanisms in nanocrystalline alloys. Mater. Sci. Eng. A 2008, 493, 3–11. [Google Scholar] [CrossRef]
- Hou, F.Q.; Li, S.J.; Hao, Y.L.; Yang, R. Nonlinear elastic deformation behaviour of Ti-30Nb-12Zr alloys. Scr. Mater. 2010, 63, 54–57. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Tong, X.; Zhang, H.; Li, D.Y. Effect of Annealing Treatment on Mechanical Properties of Nanocrystalline α-iron: an Atomistic Study. Sci. Rep. 2015, 5, 8459. [Google Scholar] [CrossRef] [PubMed]
- Billi, F.; Onofre, E.; Ebramzadeh, E.; Palacios, T.; Escudero, M.L.; Garcia-Alonso, M.C. Characterization of modified Ti6Al4V alloy after fretting-corrosion tests using near-field microscopy. Surf. Coat. Technol. 2012, 212, 134–144. [Google Scholar] [CrossRef] [Green Version]
- Baboian, R. Corrosion Tests and Standards: Application and I nterpretation, 2nd ed.; ASTM International: West Conshohocken, PA, USA, 2005. [Google Scholar]
- Mansfeld, F. The Polarization Resistance Technique for Measuring Corrosion Currents; In Mars, G., Fontana, R.W.S., Eds.; Springer: New York, NY, USA, 1976; Volume 6. [Google Scholar]
- Kaibara, Y.; Sugata, K.; Tachiki, M.; Umezawa, H.; Kawarada, H. Control wettability of the hydrogen-terminated diamond surface and the oxidized diamond surface using an atomic force microscope. Diam. Relat. Mater. 2003, 12, 560–564. [Google Scholar] [CrossRef]
- Van Kooten, T.G.; Schakenraad, J.M.; van der Mei, H.C.; Busscher, H.J. Influence of substratum wettability on the strength of adhesion of human fibroblasts. Biomaterials 1992, 13, 897–904. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Zhang, C.; Liao, Z.; Liu, W. The improvement of wettability, biotribological behavior and corrosion resistance of titanium alloy pretreated by thermal oxidation. Tribol. Int. 2014, 79, 174–182. [Google Scholar] [CrossRef]
- Bartell, F.E.; Shepard, J.W. Surface roughness as related to hysteresis of contact angles. I. The system paraffin-water-air. J. Phys. Chem. 1953, 57, 211–215. [Google Scholar] [CrossRef]
- Berg, J.M.; Eriksson, L.G.T.; Claesson, P.M.; Borve, K.G.N. Three-Component Langmuir-Blodgett Films with a Controllable Degree of Polarity. Langmuir 1994, 10, 1225–1234. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Abidi, N.; Cabrales, L. Wettability and Surface Free Energy of Graphene Films. Langmuir 2009, 25, 11078–11081. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ao, H.; Wang, Y.; Lin, W.; Yang, S.; Zhang, S.; Yu, Z.; Tang, T. Cytocompatibility with osteogenic cells and enhanced in vivo anti-infection potential of quaternized chitosan-loaded titania nanotubes. Bone Res. 2016, 4, 16027. [Google Scholar] [CrossRef] [PubMed]
- Wall, I.; Donos, N.; Carlqvist, K.; Jones, F.; Brett, P. Modified titanium surfaces promote accelerated osteogenic differentiation of mesenchymal stromal cells in vitro. Bone 2009, 45, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.; Steinmüller-nethl, D.; Goriwoda, W.; Rasse, M. Composition and Modifications of Dental Implant Surfaces. J. Oral Implant. 2015, 2015, 14. [Google Scholar] [CrossRef]
- Sullivan, D.Y.; Sherwood, R.L.; Mai, T.N. Preliminary results of a multicenter study evaluating a chemically enhanced surface for machined commercially pure titanium implants. J. Prosthet. Dent. 1997, 78, 379–386. [Google Scholar] [CrossRef]
- Conradi, M.; Kocijan, A.; Zorko, M.; Verpoest, I. Damage resistance and anticorrosion properties of nanosilica-filled epoxy-resin composite coatings. Prog. Org. Coat. 2015, 80, 20–26. [Google Scholar] [CrossRef]
- Toloei, A.S.; Stoilov, V.; Northwood, D.O. The effect of different surface topographies on the corrosion behaviour of nickel. WIT Trans. Eng. Sci. 2013, 77, 193–204. [Google Scholar]
- Burstein, G.T.; Vines, S.P. Repetitive nucleation of corrosion pits on stainless steel and the effects of surface roughness. J. Electrochem. Soc. 2001, 148, B504–B516. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Lee, B.C.; Kim, T.N.; Panigrahi, B.B. Corrosion Behaviour of Ultra Fine Grained Titanium in Simulated Body Fluid for Implant Application. Trends Biomater. Artif. Organs 2008, 22, 58–64. [Google Scholar]
- Liang, C.-H.; Mou, Z. Effects of different simulated fluids on anticorrosion biometallic materials. Trans. Nonferr. Met. Soc. China 2001, 11, 579–582. [Google Scholar]
- Narayanan, T.S.N.S. Surface Modification of Magnesium and its Alloys for Biomedical Applications; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Webb, K.; Hlady, V.; Tresco, P.A. Relative importance of surface wettability and charged functional groups on NIH 3T3 fibroblast attachment, spreading, and cytoskeletal organization. J. Biomed. Mater. Res. 1998, 41, 422–430. [Google Scholar] [CrossRef]
- Baier, R.E.; Shafrin, E.G.; Zisman, W.A. Adhesion: Mechanisms that assist or impede it. Science 1968, 162, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Raines, A.L.; Wieland, M.; Schwartz, Z.; Boyan, B.D. Requirement for both micron- and submicron scale structure for synergistic responses of osteoblasts to substrate surface energy and topography. Biomaterials 2007, 28, 2821–2829. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; McCloy, D.; Robertson, M.; Wilkinson, C.D.W.; Oreffo, R.O.C. Osteoprogenitor response to defined topographies with nanoscale depths. Biomaterials 2006, 27, 1306–1315. [Google Scholar] [CrossRef] [PubMed]














| Reagents | Simulated Body Fluid (SBF) | Hank solution |
|---|---|---|
| NaCl | 8.035 g·L−1 | 8 g·L−1 |
| NaHCO3 | 0.335 g·L−1 | - |
| KCl | 0.225 g·L−1 | 0.4 g·L−1 |
| K2HPO4·3H2O | 0.231 g·L−1 | - |
| 1 M HCl | 40 cm3 | - |
| MgCl2·6H2O | 0.311 g·L−1 | 0.1 g·L−1 |
| CaCl2 | 0.292 g·L−1 | 0.14 g·L−1 |
| Na2SO4 | 0.072 g·L−1 | - |
| (HOCH2)3CNH2 | 6.228 g·L−1 | - |
| glucose | - | 1 g·L−1 |
| NaHCO3 | - | 0.35 g·L−1 |
| NaH2PO4·6H2O | - | 0.06 g·L−1 |
| KH2PO4 | - | 0,06 g·L−1 |
| MgSO4 | - | 0,06 g·L−1 |
| pH | 7.2 | 7.5 |
| Surface Tension (mJ/m2) | Deionized Water | Ethylene Glycol | Di-Iodomethane |
|---|---|---|---|
| γLtot | 72.8 | 48.0 | 50.8 |
| γLd | 21.8 | 29.0 | 50.8 |
| γLp | 51.0 | 19.0 | 0 |
| Alloy | Ra (SD) (nm) | Rq (SD) (nm) | Ssk (SD) | H (SD) (GPa) | E (SD) (GPa) | (SD) (MPa) | (SD) (MPa) | (SD) (%) |
|---|---|---|---|---|---|---|---|---|
| T1 | 41.9 (±3.3) | 53.8 (±4.1) | −0.3 (±0.08) | 3.3 (±0.2) | 110 (±3.4) | 1026.8 (±12.1) | 929.3 (±10.8) | 11.3 (±0.8) |
| T2 | 39.1 (±2.9) | 51.6 (±4.0) | 0.2 (±0.06) | 2.6 (±0.2) | 57.4 (±2.1) | 887.4 (±56.1) | 427.2 (±31.1) | 47.6 (±6.1) |
| T3 | 41.2 (±0.4) | 51.2 (±0.4) | 0.3 (±0.03) | 4.4 (±0.1) | 52.3 (±2.0) | 765.7 (±115.2) | 488.6 (±44.7) | 2.0 (±0.4) |
| Alloy | Corrosive Solution | Ecorr (mV) | icorr (nA/cm2) | Rp (kΩ) | CR (µm/Year) |
|---|---|---|---|---|---|
| T1 | SBF | −209 | 70.41 | 802.91 | 0. 64 |
| T2 | −138 | 334.89 | 243.30 | 3.06 | |
| T3 | −150 | 68.07 | 775.6 | 0. 62 | |
| T1 | Hank | −268 | 53.12 | 855.8 | 0. 49 |
| T2 | −155 | 46.25 | 1524.6 | 0. 42 | |
| T3 | −156 | 99.56 | 687.6 | 0. 91 |
| Alloys | Contact Angle θ, ° | Surface Tension Parameters | Wadhwater (mN/m) | ||||
|---|---|---|---|---|---|---|---|
| Deionized Water | Ethylene Glycol | Di-Iodomethane | (mN/m) | (mN/m) | (mN/m) | ||
| T1 | 64.8 ± 2.6 | 48.9 ± 2.3 | 47.86 ± 1.0 | 42.7 | 10.1 | 32.6 | 98.7 |
| T2 | 57.7 ± 2.6 | 57.4 ± 3.7 | 48.6 ± 1.4 | 42.1 | 11.5 | 30.7 | 100.3 |
| T3 | 54.2 ± 5.5 | 34.14 ± 3.1 | 36.0 ± 3.2 | 49.9 | 12.4 | 37.6 | 107.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotrut, C.M.; Parau, A.C.; Gherghilescu, A.I.; Titorencu, I.; Pana, I.; Cojocaru, D.V.; Pruna, V.; Constantin, L.; Dan, I.; Vranceanu, D.M.; et al. Mechanical, In Vitro Corrosion Resistance and Biological Compatibility of Cast and Annealed Ti25Nb10Zr Alloy. Metals 2017, 7, 86. https://doi.org/10.3390/met7030086
Cotrut CM, Parau AC, Gherghilescu AI, Titorencu I, Pana I, Cojocaru DV, Pruna V, Constantin L, Dan I, Vranceanu DM, et al. Mechanical, In Vitro Corrosion Resistance and Biological Compatibility of Cast and Annealed Ti25Nb10Zr Alloy. Metals. 2017; 7(3):86. https://doi.org/10.3390/met7030086
Chicago/Turabian StyleCotrut, Cosmin M., Anca C. Parau, Ana I. Gherghilescu, Irina Titorencu, Iulian Pana, Danut V. Cojocaru, Vasile Pruna, Lidia Constantin, Ioan Dan, Diana M. Vranceanu, and et al. 2017. "Mechanical, In Vitro Corrosion Resistance and Biological Compatibility of Cast and Annealed Ti25Nb10Zr Alloy" Metals 7, no. 3: 86. https://doi.org/10.3390/met7030086
APA StyleCotrut, C. M., Parau, A. C., Gherghilescu, A. I., Titorencu, I., Pana, I., Cojocaru, D. V., Pruna, V., Constantin, L., Dan, I., Vranceanu, D. M., & Vladescu, A. (2017). Mechanical, In Vitro Corrosion Resistance and Biological Compatibility of Cast and Annealed Ti25Nb10Zr Alloy. Metals, 7(3), 86. https://doi.org/10.3390/met7030086








.jpg)