Effect of Carbon Content on the Microstructure and Mechanical Properties of NbC-Ni Based Cermets
Abstract
:1. Introduction
2. Experimental
2.1. Materials Preparation
2.2. Characterization
3. Results and Discussion
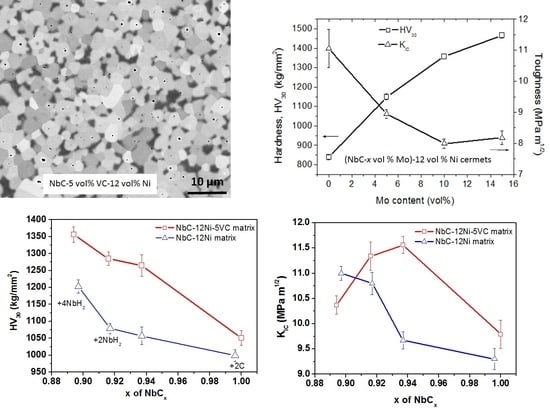
3.1. Influence of Carbon Content in NbC-12 vol % Ni Cermets
3.2. Influence of the Carbon Content in NbC-12 Ni-5 VC (vol %) Cermets
3.3. Influence of Mo Content in Carbon-Rich NbC-12 vol % Ni Cermets
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bock, A.; Schubert, W.D.; Lux, B. Inhibition of Grain Growth on Submicron Cemented Carbides. Powder Metall. Int. 1992, 24, 20–26. [Google Scholar]
- Sadangi, R.K.; McCandlish, L.E.; Kear, B.H.; Seegopaul, P. Grain Growth Inhibition in Liquid Phase Sintered Nanophase WC/Co Alloys. Int. J. Powder Met. 1999, 35, 27–33. [Google Scholar]
- Wittmann, B.; Schubert, W.D.; Lux, B. WC Grain Growth and Grain Growth Inhibition in Nickel and Iron Binder Hardmetals. Int. J. Refract. Met. Hard Mater. 2002, 20, 51–60. [Google Scholar] [CrossRef]
- Delannay, F.; Froyen, L.; Deruyttere, A. The Wetting of Solids by Molten Metals and its Relation to the Preparation of Metal-Matrix Composites. J. Mater. Sci. 1987, 22, 1–16. [Google Scholar] [CrossRef]
- Zhang, S. Titanium Carbonitride-Based Cermets: Processes and Properties. Mater. Sci. Eng. A 1993, 163, 141–148. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Kim, S.W.; Kang, S. Microstructure of Ti(CN)-WC-NbC-Ni Cermets. J. Am. Ceram. Soc. 2001, 84, 843–849. [Google Scholar] [CrossRef]
- Peng, Y.; Miao, H.Z.; Peng, Z.J. Development of TiCN-Based Cermets: Mechanical Properties and Wear Mechanism. Int. J. Refract. Met. Hard Mater. 2013, 39, 79–89. [Google Scholar] [CrossRef]
- Pierson, H.O. Handbook of Refractory Carbides and Nitrides; William Andrew: Noyes, NJ, USA, 1996. [Google Scholar]
- Woydt, M.; Mohrbacher, H. The Tribological and Mechanical Properties of Niobium Carbides (NbC) Bonded with Cobalt or Fe3Al. Wear 2014, 321, 1–7. [Google Scholar] [CrossRef]
- Huang, S.G.; Vleugels, J.; Mohrbacher, H.; Woydt, M. Microstructure and Tribological Performance of NbC-Ni Cermets Modified by VC and Mo2C. Int. J. Refract. Met. Hard Mater. 2017, 66, 188–197. [Google Scholar] [CrossRef]
- Kropidlowski, K.; Uhlmann, E.; Woydt, M. Außen-Längs-Runddrehen mit Niobcarbid-Schneidstoff (Straight turning with cutting tools in niobium carbide). BAM 2017, 7–8, 58–61. (In German) [Google Scholar]
- Huang, S.G.; Li, L.; Van der Biest, O.; Vleugels, J. Influence of WC Addition on the Microstructure and Mechanical Properties of NbC-Co Cermets. J. Alloys Compd. 2007, 430, 158–164. [Google Scholar] [CrossRef]
- Huang, S.G.; Li, L.; Van der Biest, O.; Vleugels, J. VC and Cr3C2 doped WC-NbC-Co Hardmetals. J. Alloys Compd. 2008, 464, 203–209. [Google Scholar] [CrossRef]
- Huang, S.G.; Vanmeensel, K.; Mohrbacher, H.; Woydt, M.; Vleugels, J. Microstructure and Mechanical Properties of NbC-Matrix Hardmetals with Secondary Carbide Addition and Different Metal Binders. Int. J. Refract. Met. Hard Mater. 2015, 48, 418–426. [Google Scholar] [CrossRef]
- Huang, S.G.; Vleugels, J.; Mohrbacher, H.; Woydt, M. Microstructure and Mechanical Properties of NbC Matrix Cermets Using Ni Containing Metal Binder. Met. Powder Rep. 2016, 71, 349–355. [Google Scholar] [CrossRef]
- Ramqvist, L. Variation of Hardness, Resistivity, and Lattice Parameter with Carbon Content of Group 5 b Metal Carbides. Jernkontorets Ann. 1968, 152, 465–475. [Google Scholar]
- Vinitskii, I.M. Relation between the properties of groups IV-V transition metals and carbon content. Powder Metall. Met. Ceram. 1972, 11, 488–493. [Google Scholar] [CrossRef]
- Delanoë, A.; Lay, S. Evolution of the WC Grain Shape in WC-Co Alloys during Sintering: Effect of the C Content. Int. J. Refract. Met. Hard Mater. 2009, 27, 140–148. [Google Scholar] [CrossRef]
- Zackrisson, J.; Andrén, H.-O. Effect of Carbon Content on the Microstructure and Mechanical Properties of (Ti, W, Ta, Mo)(C, N)-(Co, Ni) Cermets. Int. J. Refract. Met. Hard Mater. 1999, 17, 265–273. [Google Scholar] [CrossRef]
- Kang, S. Stability of N in Ti(CN) Solid Solutions for Cermet Applications. Powder Metall. 1997, 40, 139–142. [Google Scholar] [CrossRef]
- Kenneth, C.; Russell, S.Y.; Figueredo, A. Theoretical and Experimental Studies of Ceramic: Metal Wetting. MRS Bull. 1991, 4, 46–52. [Google Scholar]
- Liu, N.; Liu, X.S.; Zhang, X.B. Effect of Carbon Content on the Microstructure and Mechanical Properties of Superfine Ti(C, N)-based Cermets. Mater. Charact. 2008, 59, 1440–1446. [Google Scholar] [CrossRef]
- Shetty, D.K.; Wright, I.G.; Mincer, P.N.; Clauer, A.H. Indentation Fracture of WC-Co Cermets. J. Mater. Res. 1985, 20, 1873–1882. [Google Scholar] [CrossRef]
- Warren, R. Carbide Grain Growth During the Liquid-Phase Sintering of the Alloys NbC-Fe, NbC-Ni, and NbC-Co. J. Less-Common Met. 1969, 17, 65–72. [Google Scholar] [CrossRef]
- Gabriel, S.B.; Brum, M.C.; Candioto, K.C.G.; Sandim, H.R.Z.; Suzuki, P.A.; Nunes, C.A. Kinetics of thermal decomposition of niobium hydride. Int. J. Refract. Met. Hard Mater. 2012, 30, 38–41. [Google Scholar] [CrossRef] [Green Version]
- Storms, E.K.; Krikorian, N.H. The Variation of Lattice Parameter with Carbon Content of Niobium Carbide. J. Phys. Chem. 1959, 63, 1747–1749. [Google Scholar] [CrossRef]
- Sundman, B.; Jansson, B.; Andersson, J.O. The Thermo-Calc Databank System. Calphad 1985, 9, 153–190. [Google Scholar] [CrossRef]
- Lay, S.; Hamar-Thibault, S.; Lackner, A. Location of VC in VC, Cr3C2 Codoped WC-Co Cermets by HREM and EELS. Int. J. Refract. Met. Hard Mater. 2002, 20, 61–69. [Google Scholar] [CrossRef]
- Egami, M.E.; Machida, M. Morphology of Vanadium Carbide in Submicron Hardmetals. In Proceedings of the 13th International Plansee Seminar, Plansee AG, Wattens, Austria, 24–28 May 1993; pp. 639–648. [Google Scholar]
- Edwards, R.; Raine, T. The Solid Solubility of Some Stable Carbides in Cobalt Nickel and Iron at 1250 °C. Pulvermetallurgie Springerverlag 1952, 232–242. [Google Scholar]









| Powder | Density (g/cm3) | Particle Sized50 (μm) | Total C Content(wt %) | Total O Content(wt %) |
|---|---|---|---|---|
| NbC-A | 7.71 | 9.4 | 11.2 | 0.28 |
| NbC-B | 7.46 | 6.9 | 12.3 | 0.37 |
| Experimental Goal | Composition (vol %) | Calculated C in Carbide (wt %) | Starting Powders |
|---|---|---|---|
| Influence of C on NbC-12 vol % Ni cermets | (NbC-4NbH2)-12Ni | 10.82 | NbC-A, Ni, NbH2, C |
| (NbC-2NbH2)-12Ni | 11.01 | ||
| NbC-12Ni | 11.20 | ||
| (NbC-2C)-12Ni | 11.72 | ||
| (NbC-4C)-12Ni | 12.26 | ||
| Influence of C on NbC-12 vol % Ni-5 vol % VC cermets | (NbC-4NbH2)-5VC-12Ni | 11.11 | NbC-A, Ni, NbH2, C |
| (NbC-2NbH2)-5VC-12Ni | 11.30 | ||
| NbC-5VC-12Ni | 11.50 | ||
| (NbC-2C)-5VC-12Ni | 12.03 | ||
| Influence of Mo on C-rich NbC-12 vol % Ni cermets | NbC-12Ni | 12.20 | NbC-B, Ni, Mo |
| (NbC-5Mo)-12Ni | 11.41 | ||
| (NbC-10Mo)-12Ni | 10.64 | ||
| (NbC-15Mo)-12Ni | 9.90 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Baets, P.D.; Sukumaran, J.; Mohrbacher, H.; Woydt, M.; Vleugels, J. Effect of Carbon Content on the Microstructure and Mechanical Properties of NbC-Ni Based Cermets. Metals 2018, 8, 178. https://doi.org/10.3390/met8030178
Huang S, Baets PD, Sukumaran J, Mohrbacher H, Woydt M, Vleugels J. Effect of Carbon Content on the Microstructure and Mechanical Properties of NbC-Ni Based Cermets. Metals. 2018; 8(3):178. https://doi.org/10.3390/met8030178
Chicago/Turabian StyleHuang, Shuigen, Patrick De Baets, Jacob Sukumaran, Hardy Mohrbacher, Mathias Woydt, and Jozef Vleugels. 2018. "Effect of Carbon Content on the Microstructure and Mechanical Properties of NbC-Ni Based Cermets" Metals 8, no. 3: 178. https://doi.org/10.3390/met8030178
APA StyleHuang, S., Baets, P. D., Sukumaran, J., Mohrbacher, H., Woydt, M., & Vleugels, J. (2018). Effect of Carbon Content on the Microstructure and Mechanical Properties of NbC-Ni Based Cermets. Metals, 8(3), 178. https://doi.org/10.3390/met8030178