Synthesis of Spherical V-Nb-Mo-Ta-W High-Entropy Alloy Powder Using Hydrogen Embrittlement and Spheroidization by Thermal Plasma
Abstract
:1. Introduction
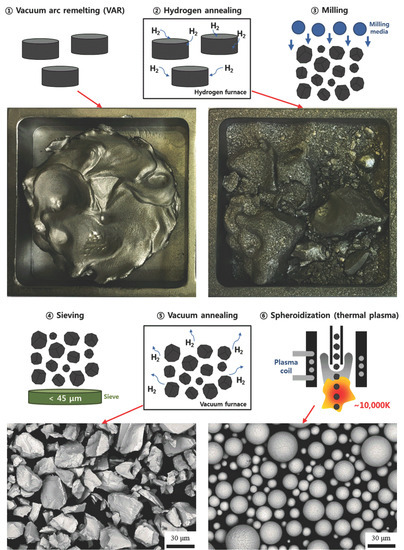
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Youssef, K.M.; Zaddach, A.J.; Niu, C.; Irving, D.L.; Koch, C.C. A novel low-density, high-hardness, high-entropy alloy with close-packed single-phase nanocrystalline structures. Mater. Res. Lett. 2015, 3, 95–99. [Google Scholar] [CrossRef]
- Yu, P.F.; Zhang, L.J.; Cheng, H.; Zhang, H.; Ma, M.Z.; Li, Y.C.; Li, G.; Liaw, P.K.; Liu, R.P. The high-entropy alloys with high hardness and soft magnetic property prepared by mechanical alloying and high-pressure sintering. Intermetallics 2016, 70, 82–87. [Google Scholar] [CrossRef]
- Yeh, J.; Chen, S.; Gan, J.; Lin, S.; Chin, T. Formation of simple crystal structures in Cu-Co-Ni-Cr-Al-Fe-Ti-V alloys with multiprincipal metallic elements. Metall. Mater. Trans. A 2010, 35, 2533–2536. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, Y.; Wang, Y.L.; Chen, G.L. Solid solution alloys of AlCoCrFeNiTix with excellent room-temperature. Appl. Phys. Lett. 2014, 90, 181904. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, W.; Fang, S.; Zhang, D.; Xiao, H.; Zhu, D. Alloying behavior and deformation twinning in a CoNiFeCrAl0. 6Ti0. 4 high entropy alloy processed by spark plasma sintering. J. Alloys Compd. 2013, 553, 316–323. [Google Scholar] [CrossRef]
- Chou, H.P.; Chang, Y.S.; Chen, S.K.; Yeh, J.W. Microstructure, thermophysical and electrical properties in AlxCoCrFeNi (0 ≤ x ≤ 2) high-entropy alloys. Mater. Sci. Eng. B. 2009, 163, 184–189. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, H.; Wu, Y.; Liu, X.; Lu, Z. Thermoelectric performance of PbSnTeSe high-entropy alloys. Mater. Res. Lett. 2017, 5, 187–194. [Google Scholar] [CrossRef]
- Wu, W.H.; Yang, C.C.; Yeh, L.W. Industrial development of high-entropy alloys. Ann. Chim. Des Mater. 2006, 31, 737–747. [Google Scholar] [CrossRef]
- Schuh, B.; Mendez-Martin, F.; Völker, B.; George, E.P.; Clemens, H.; Pippan, R.; Hohenwarter, A. Mechanical properties, microstructure and thermal stability of a nanocrystalline CoCrFeMnNi high-entropy alloy after severe plastic deformation. Acta Mater. 2015, 96, 258–268. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Shaysultanov, D.G.; Salishchev, G.A.; Tikhonovsky, M.A. Structure and mechanical properties of a light-weight AlNbTiV high entropy alloy. Mater. Lett. 2015, 142, 153–155. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Duval, T.; Hung, U.D.; Yeh, J.W.; Shih, H.C. Microstructure and electrochemical properties of high entropy alloys—A comparison with type-304 stainless steel. Corros. Sci. 2005, 47, 2257–2279. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Chiang, W.C.; Wu, J.K. Corrosion behavior of FeCoNiCrCux high-entropy alloys in 3.5% sodium chloride solution. Mater. Chem. Phys. 2005, 92, 112–117. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Liaw, P. Corrosion-resistant high-entropy alloys: A review. Metals 2017, 7, 43. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Yeh, J.W.; Chen, S.K.; Shun, T.T. Wear resistance and high-temperature compression strength of Fcc CuCoNiCrAl0.5Fe alloy with boron addition. Metall. Mater. Trans. A 2004, 35, 1465–1469. [Google Scholar] [CrossRef]
- Chuang, M.H.; Tsai, M.H.; Wang, W.R.; Lin, S.J.; Yeh, J.W. Microstructure and wear behavior of AlxCo1. 5CrFeNi1. 5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W.; Gan, J.Y. Diffusion barrier properties of AlMoNbSiTaTiVZr high-entropy alloy layer between copper and silicon. Thin Solid Films 2008, 516, 5527–5530. [Google Scholar] [CrossRef]
- Chang, S.Y.; Wang, C.Y.; Chen, M.K.; Li, C.E. Ru incorporation on marked enhancement of diffusion resistance of multi-component alloy barrier layers. J. Alloys Compd. 2011, 509, L85–L89. [Google Scholar] [CrossRef]
- Han, Z.D.; Chen, N.; Zhao, S.F.; Fan, L.W.; Yang, G.N.; Shao, Y.; Yao, K.F. Effect of Ti additions on mechanical properties of NbMoTaW and VNbMoTaW refractory high entropy alloys. Intermetallics 2017, 84, 153–157. [Google Scholar] [CrossRef]
- Xin, S.W.; Zhang, M.; Yang, T.T.; Zhao, Y.Y.; Sun, B.R.; Shen, T.D. Ultrahard bulk nanocrystalline VNbMoTaW high-entropy alloy. J. Alloys Compd. 2018, 769, 597–604. [Google Scholar] [CrossRef]
- Nam, H.; Park, C.; Kim, C.; Kim, H.; Kang, N. Effect of post weld heat treatment on weldability of high entropy alloy welds. Sci. Technol. Weld. Join. 2018, 23, 420–427. [Google Scholar] [CrossRef]
- Thompson, S.M.; Bian, L.; Shamsaei, N.; Yadollahi, A. An overview of Direct Laser Deposition for additive manufacturing; Part I: Transport phenomena, modeling and diagnostics. Addit. Manuf. 2015, 8, 36–62. [Google Scholar] [CrossRef]
- Todd, I.; Sidambe, A.T. Developments in metal injection moulding (MIM). In Advances in Powder Metallurgy; Woodhead Publishing: Sawston, UK, 2013; pp. 109–146. [Google Scholar]
- Robertson, I.M.; Sofronis, P.; Nagao, A.; Martin, M.L.; Wang, S.; Gross, D.W.; Nygren, K.E. Hydrogen embrittlement understood. Metall. Mater. Trans. A 2015, 46, 2323–2341. [Google Scholar] [CrossRef]
- Popov, B.N.; Lee, J.W.; Djukic, M.B. Hydrogen permeation and hydrogen-induced cracking. In Handbook of Environmental Degradation of Materials; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 133–162. [Google Scholar]
- Djukic, M.B.; Bakic, G.M.; Zeravcic, V.S.; Sedmak, A.; Rajicic, B. The synergistic action and interplay of hydrogen embrittlement mechanisms in steels and iron: localized plasticity and decohesion. Eng. Fract. Mech. 2019, 216, 106528. [Google Scholar] [CrossRef]
- Ichii, K.; Koyama, M.; Tasan, C.C.; Tsuzaki, K. Comparative study of hydrogen embrittlement in stable and metastable high-entropy alloys. Scr. Mater. 2018, 150, 74–77. [Google Scholar] [CrossRef]
- Zhao, Y.K.; Lee, D.H.; Seok, M.Y.; Lee, J.A.; Phaniraj, M.P.; Suh, J.Y.; Ha, H.Y.; Kim, J.Y.; Ramamurty, U.; Jang, J.I. Resistance of CoCrFeMnNi high-entropy alloy to gaseous hydrogen embrittlement. Scr. Mater. 2017, 135, 54–58. [Google Scholar] [CrossRef]
- Nygren, K.E.; Wang, S.; Bertsch, K.M.; Bei, H.B.; Nagao, A.; Robertson, I.M. Hydrogen embrittlement of the equi-molar FeNiCoCr alloy. Acta Mater. 2018, 157, 218–227. [Google Scholar] [CrossRef]
- Luo, H.; Li, Z.M.; Lu, W.J.; Ponge, D.; Raabe, D. Hydrogen embrittlement of an interstitial equimolar high-entropy alloy. Corros. Sci. 2018, 136, 403–408. [Google Scholar] [CrossRef]
- Moreen, H.A.; Taggart, R.; Polonis, D.H. A model for the prediction of lattice parameters of solid solutions. Metall. Trans. 1971, 2, 265–268. [Google Scholar] [CrossRef]
- Owen, C.V.; Scott, T.E. Relation between hydrogen embrittlement and the formation of hydride in the group V transition metals. Metall. Mater. Trans. B 1972, 3, 1715–1726. [Google Scholar] [CrossRef]
- Park, K.B.; Park, J.M.; Choi, J.; Kang, J.-W.; Lee, S.Y.; Park, K.; Lee, T.-W.; Na, T.-W.; Park, H.-K. Preparation of Nb-silicide based alloy powder by hydrogenation-dehydrogenation reaction. Int. J. Refract. Met. Hard Mater. 2018, 76, 180–184. [Google Scholar] [CrossRef]
- Cotterill, P. The hydrogen embrittlement of metals. Prog. Mater. Sci. 1961, 9, 205–301. [Google Scholar] [CrossRef]
- Azevedo, C.R.F.; Rodrigues, D.; Neto, F.B. Ti-Al-V powder metallurgy (PM) via the hydrogenation--dehydrogenation (HDH) process. J. Alloys Compd. 2003, 353, 217–227. [Google Scholar] [CrossRef]
- Oh, J.M.; Roh, K.M.; Lee, B.K.; Suh, C.Y.; Kim, W.; Kwon, H.; Lim, J.W. Preparation of low oxygen content alloy powder from Ti binary alloy scrap by hydrogenation-dehydrogenation and deoxidation process. J. Alloys Compd. 2014, 593, 61–66. [Google Scholar] [CrossRef]
- Boulos, M.I. The role of transport phenomena and modeling in the development of thermal plasma technology. Plasma Chem. Plasma Process. 2016, 36, 3–28. [Google Scholar] [CrossRef]
- Grant, N.J. Rapid solidification of metallic particulates. JOM 1983, 35, 20–27. [Google Scholar] [CrossRef] [Green Version]










| Element | Ta | W | Nb | Mo | V |
|---|---|---|---|---|---|
| Atomic% | 21.07 | 19.75 | 19.3 | 20.39 | 19.49 |
| Sample | V | Nb | Mo | Ta | W | ||
|---|---|---|---|---|---|---|---|
| Ingot | Dendrite arm | wt% | 6.00 | 14.43 | 15.60 | 30.86 | 33.11 |
| at% | 14.97 | 19.75 | 20.68 | 21.34 | 23.26 | ||
| Interdendritic region | wt% | 18.61 | 19.12 | 17.63 | 27.46 | 17.19 | |
| at% | 36.56 | 20.59 | 18.39 | 14.95 | 9.51 | ||
| Spheroidized powder | Dendrite arm | wt% | 4.00 | 12.52 | 13.73 | 30.55 | 39.21 |
| at% | 10.62 | 18.23 | 19.35 | 22.48 | 29.31 | ||
| Interdendritic region | wt% | 17.04 | 24.31 | 19.84 | 22.47 | 16.34 | |
| at% | 32.94 | 25.76 | 20.37 | 12.03 | 8.89 | ||
| Sample | Composition | Oxygen | Nitrogen | Hydrogen |
|---|---|---|---|---|
| Ingot | wt% | 0.065 | 0.004 | 0.0009 |
| at% | 0.488 | 0.034 | 0.1074 | |
| Hydrogen-annealed powder | wt% | 0.066 | 0.011 | 0.1033 |
| at% | 0.442 | 0.084 | 11.0634 | |
| Vacuum-annealed powder | wt% | 0.067 | 0.004 | 0.0002 |
| at% | 0.504 | 0.034 | 0.0241 | |
| Spheroidized powder | wt% | 0.042 | 0.005 | 0.0003 |
| at% | 0.316 | 0.043 | 0.0359 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-H.; Park, K.B.; Yi, K.-W.; Lee, S.Y.; Park, K.; Lee, T.W.; Na, T.-W.; Park, H.-K. Synthesis of Spherical V-Nb-Mo-Ta-W High-Entropy Alloy Powder Using Hydrogen Embrittlement and Spheroidization by Thermal Plasma. Metals 2019, 9, 1296. https://doi.org/10.3390/met9121296
Lee W-H, Park KB, Yi K-W, Lee SY, Park K, Lee TW, Na T-W, Park H-K. Synthesis of Spherical V-Nb-Mo-Ta-W High-Entropy Alloy Powder Using Hydrogen Embrittlement and Spheroidization by Thermal Plasma. Metals. 2019; 9(12):1296. https://doi.org/10.3390/met9121296
Chicago/Turabian StyleLee, Won-Hyuk, Ki Beom Park, Kyung-Woo Yi, Sung Yong Lee, Kwangsuk Park, Taeg Woo Lee, Tae-Wook Na, and Hyung-Ki Park. 2019. "Synthesis of Spherical V-Nb-Mo-Ta-W High-Entropy Alloy Powder Using Hydrogen Embrittlement and Spheroidization by Thermal Plasma" Metals 9, no. 12: 1296. https://doi.org/10.3390/met9121296
APA StyleLee, W. -H., Park, K. B., Yi, K. -W., Lee, S. Y., Park, K., Lee, T. W., Na, T. -W., & Park, H. -K. (2019). Synthesis of Spherical V-Nb-Mo-Ta-W High-Entropy Alloy Powder Using Hydrogen Embrittlement and Spheroidization by Thermal Plasma. Metals, 9(12), 1296. https://doi.org/10.3390/met9121296