Complex Precipitates of TiN-MCx in GCr15 Bearing Steel
Abstract
:1. Introduction
2. Experiment
2.1. Chemical Components Analysis
2.2. Non-Aqueous Electrolysis and XRD Detection
3. Result
3.1. Observation for Particles
3.2. XRD Result
4. Discussion
4.1. Thermodynamic Analysis
4.2. Crystallographic Analysis
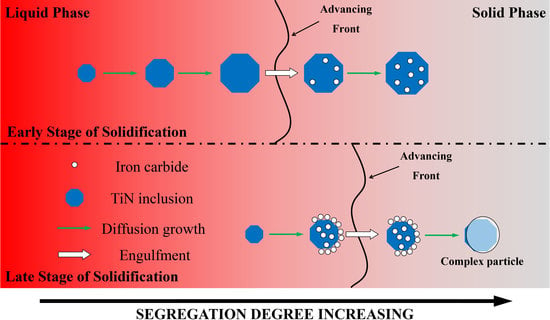
4.3. Pushing and Engulfment Behavior of Particles
- TiN-MCx with high TiN volume fraction precipitates at the early stage of solidification and has better growth kinetics in the melt. After being engulfed by the solidification front, MCx grows at a lower rate on the surface of TiN.
- TiN-MCx with low TiN volume fraction precipitates in the late stage of solidification and does not have enough time to grow to large size. Due to high C concentration and segregation, a large amount of MCx precipitates on TiN surface. When TiN-MCx is large enough and engulfed by the solidification front, the volume fraction of MCx is large enough to cover the TiN particle.
5. Conclusions
- (1)
- TiN-MCxcomposed of TiN and MCx, TiN is the effective heterogeneous nucleation site for Fe7C3 and Fe3C, in which the MCx precipitates on the surface of TiN was observed in GCr15 bearing steel.
- (2)
- MCx(M = Fe, Cr, Mn) in GCr15 bearing steel smelted by converter is mainly composed of M3C, M7C3, and Cr3C2.
- (3)
- TiN-MCx with high TiN volume fraction precipitates at the early solidification stage. After being engulfed by the solidification front, MCx grows at a lower rate on the surface of TiN.
- (4)
- TiN-MCxwith low TiN volume fraction precipitates in the late solidification stage and does not have enough time to grow to large size. When the size of TiN-MCx is large enough and is engulfed by the solidification front, the volume fraction of MCx is large enough to cover TiN particle because of high C concentration and segregation.
Author Contributions
Funding
Conflicts of Interest
References
- Beswick, J.M. The effect of chromium in high carbon bearing steels. Metall. Trans. A 1987, 18, 1897–1906. [Google Scholar] [CrossRef]
- Du, G.; Li, J.; Wang, Z.B. Control of carbide precipitation during electroslag remelting-continuous rapid solidification of GCr15 steel. Metall. Mater. Trans. B 2017, 48, 2873–2890. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, G.G.; Li, S.J.; Zhao, M.; Feng, G.P. Generation mechanism of TiN inclusion for GCr15SiMn during electroslag remelting process. ISIJ Int. 2015, 55, 1901–1905. [Google Scholar] [CrossRef]
- Zhou, D.G.; Fu, J.; Chen, X.C.; Li, J. Precipitation behavior of TiN in bearing steel. J. Mater. Sci. Technol. 2003, 19, 184–186. [Google Scholar] [CrossRef]
- Benz, R.; Elliott, J.F.; Chipman, J. Thermodynamics of the carbides in the system Fe-Cr-C. Metall. Trans. 1974, 5, 2235–2240. [Google Scholar] [CrossRef]
- Benz, R.; Elliott, J.F.; Chipman, J. Thermodynamics of the solid phases in the system Fe-Mn-C. Metall. Trans. 1973, 4, 1975–1986. [Google Scholar] [CrossRef]
- Krauss, G. Principles of Heat Treatment of Steel; ASM: Metals Park, OH, USA, 1980; pp. 222–224. [Google Scholar]
- Monma, K.; Maruta, R.; Yamamoto, T.; Wakikado, Y. Role of spheroidized carbides on the fatigue life of bearing steel. Jpn. Inst. Metall. J. 1968, 32, 1198–1204. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, J.; Di, L.; Tong, F.; Liu, D.; Wang, Y. Study on the precipitation behavior of TiN in the microalloyed steels. Acta Metall. Sin. 2000, 36, 801–804. [Google Scholar]
- Lee, M.H.; Park, J.H. Synergistic effect of nitrogen and refractory material on TiN formation and equiaxed grain structure of ferritic stainless steel. Metall. Mater. Trans. B 2018, 49, 877–893. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, D.H.; Park, J.H. TEM characterization of a TiN-MgAl2O4 epitaxial interface. J. Alloys Comp. 2017, 695, 476–481. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.F.; Duan, H.J.; Zhang, Y.; Luo, Y.; Conejo, A.N. Extraction, thermodynamic analysis, and precipitation mechanism of MnS-TiN complex inclusions in low-sulfur steels. Metall. Mater. Trans. A 2016, 47, 3015–3025. [Google Scholar] [CrossRef]
- Lee, Y.; Cooman, B.C.D. TiN/NbC compound particle formation during thin slab direct rolling of HSLA steel. Steel Res. Int. 2014, 85, 1158–1172. [Google Scholar] [CrossRef]
- Tian, Q.R.; Wang, G.C.; Shang, D.L.; Lei, H.; Yuan, X.H.; Wang, Q.; Li, J. Precipitation behaviors of TiN inclusion in GCr15 bearing steel billet. Metall. Mater. Trans. B 2018, 49, 1149–1164. [Google Scholar] [CrossRef]
- Fang, K.M.; Ni, R.M. Research on determination of the rare-earth content in metal phases of steel. Metall. Trans. A 1986, 17, 315–323. [Google Scholar]
- Bi, Y.; Karasev, A.; Jönsson, P.G. Three-dimensional investigations of inclusions in ferroalloys. Steel Res. Int. 2014, 85, 659–669. [Google Scholar] [CrossRef]
- Wang, G.C.; Li, S.L.; Ai, X.G.; Zhao, C.M.; Lai, C.B. Characterization and thermodynamics of Al2O3-MnO-SiO2 (-MnS) inclusion formation in carbon steel billet. J. Iron Steel Res. Int. 2015, 22, 566–572. [Google Scholar] [CrossRef]
- Zhang, X.W.; Zhang, L.F.; Yang, W.; Wang, Y.; Liu, Y.; Dong, Y.C. Characterization of the three-dimensional morphology and formation mechanism of inclusions in linepipe steels. Metall. Mater. Trans. B 2017, 48, 701–712. [Google Scholar] [CrossRef]
- Zhang, X.W.; Zhang, L.F.; Yang, W.; Dong, Y.C. Characterization of MnS particles in heavy rail steels using different methods. Steel Res. Int. 2017, 88, 1600080. [Google Scholar] [CrossRef]
- Zhang, D.; Shen, P.; Xie, J.B.; An, J.M.; Huang, Z.Z.; Fu, J.X. A method for observing tridimensional morphology of sulfide inclusions by non-aqueous solution electrolytic etching. J. Iron Steel Res. Int. 2019, 26, 275–284. [Google Scholar] [CrossRef]
- Andersson, J. A thermodynamic evaluation of the Fe-Cr-C system. Metall. Trans. A 1988, 19, 627–636. [Google Scholar] [CrossRef]
- Tian, Q.R.; Wang, G.C.; Shang, D.L.; Lei, H.; Yuan, X.H.; Wang, Q.; Li, J. In Situ Observation of the Precipitation, Aggregation, and Dissolution Behaviors of TiN Inclusion on the Surface of Liquid GCr15 Bearing Steel. Metall. Mater. Trans. B 2018, 49, 3137–3150. [Google Scholar] [CrossRef]
- Li, B.; Shi, X.; Guo, H.J.; Guo, J. Study on Precipitation and Growth of TiN in GCr15 Bearing Steel during Solidification. Materials 2019, 12, 1463. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Yang, J.; Bao, Y.P. Characteristics of BN precipitation and growth during solidification of BN free-machining steel. Metall. Mater. Trans. B 2014, 45, 2269–2278. [Google Scholar] [CrossRef]
- Huang, Y.W.; Long, M.J.; Liu, P.; Chen, D.F.; Chen, H.B.; Gui, L.T.; Liu, T.; Yu, S. Effects of partition coefficients, diffusion coefficients, and solidification paths on microsegregation in Fe-based multinary alloy. Metall. Mater. Trans. B 2017, 48, 2504–2515. [Google Scholar] [CrossRef]
- Miettinen, J. Thermodynamic-kinetic simulation of constrained dendrite growth in steels. Metall. Mater. Trans. B 2000, 31, 365–379. [Google Scholar] [CrossRef]
- Ohnaka, I. Mathematical analysis of solute redistribution during solidification with diffusion in solid phase. ISIJ Int. 1986, 26, 1045–1051. [Google Scholar] [CrossRef]
- Turnbull, D.; Vonnegut, B. Nucleation catalysis. Ind. Eng. Chem. 1952, 44, 1292–1298. [Google Scholar] [CrossRef]
- Bramfitt, B.L. The effect of carbide and nitride additions on the heterogeneous nucleation behavior of liquid iron. Metall. Trans. 1970, 1, 1987–1995. [Google Scholar] [CrossRef]
- Ding, M.Y.; Yang, Y.; Wu, B.S.; Li, Y.W.; Wang, T.J.; Ma, L.L. Study on reduction and carburization behaviors of iron-based Fischer-Tropsch synthesis catalyst. Energy Procedia 2014, 61, 2267–2270. [Google Scholar] [CrossRef]
- Morniroli, J.P.; Gantois, M. Etude microstructurale de carbures M7C3. J. Appl. Crystallogr. 1983, 16, 1–10. [Google Scholar] [CrossRef]
- Fruchart, D.; Chaudouet, P.; Fruchart, R.; Rouault, A.; Senateur, J.P. Etudes structurales de composés de type cémentite: Effet de l’hydroge‘ne sur Fe3C suivi par diffraction neutronique. Spectrométrie Mössbauer sur FeCo 2B et Co 3B dopés au 57Fe. J. Solid State Chem. 1984, 51, 246–252. [Google Scholar] [CrossRef]
- Descotes, V.; Bellot, J.P.; Witzke, S.; Jardy, A. Modeling the titanium nitride (TiN) germination and growth during the solidification of a maraging steel. In Proceedings of the 2013 International Symposium on Liquid Metal Processing & Casting, Austin, TX, USA, 22–25 September 2013; pp. 201–206. [Google Scholar]
- Pervushin, G.V.; Suito, H. Precipitation behavior of TiN in Fe-10mass% Ni alloy during solidification and isothermal holding at 1400 °C. ISIJ Int. 2001, 41, 728–737. [Google Scholar] [CrossRef]
- Shibata, H.; Yin, H.; Yoshinaga, S.; Emi, T.; Suzuki, M. In-situ observation of engulfment and pushing of nonmetallic inclusions in steel melt by advancing melt/solid interface. ISIJ Int. 1998, 38, 149–156. [Google Scholar] [CrossRef]








| Composition | C | Si | Mn | P | S | Ti | Cr | V | N | Alt | Ca | O (T) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | 1.01 | 0.25 | 0.36 | 0.012 | 0.0014 | 0.0078 | 1.46 | 0.0099 | 0.0049 | 0.012 | <0.005 | 0.0009 |
| Element | Equilibrium Partition Coefficient, k | Diffusion Coefficient in γ-phase (cm2/s) |
|---|---|---|
| C | 0.34 | 0.0761·EXP (−134600/RT) |
| Cr | 0.85 | 0.0012·EXP (−219000/RT) |
| Mn | 0.78 | 0.486·EXP (−276100/RT) |
| Ti | 0.33 | 0.15·EXP (−251000/RT) |
| N | 0.48 | 0.91·EXP (−168500/RT) |
| Substance (Space Group) | Lattice Parameters (Length Unit: Å) | |||||
| Fe7C3 (Pnma) [31] | a | b | C | α = β = γ (°) | ||
| 4.537 | 6.892 | 11.913 | 90 | |||
| TiN-Fe7C3 | [hkl]s | [hkl]n | θ (°) | Disregistry | ||
| (100)TiN‖(100)Fe7C3 | 2.118 2.995 2.118 | 11.913 13.763 6.892 | 0(-) 14.949 - | 6.52% | ||
| (110)TiN‖(110)Fe7C3 | 2.995 3.668 2.118 | 11.913 14.492 8.251 | - 0.556 - | 1.49% | ||
| (111)TiN‖(111)Fe7C3 | 2.995 2.995 2.995 | 13.763 12.748 8.251 | 5.275 18.715 - | 8.93% | ||
| Substance (Space Group) | Lattice Parameters (Length Unit: Å) | |||||
| Fe3C (Pnma) [32] | a | b | C | α = β = γ (°) | ||
| 5.092 | 6.741 | 4.527 | 90 | |||
| TiN-Fe3C | [hkl]s | [hkl]n | θ (°) | Disregistry | ||
| (100)TiN‖(100)Fe3C | 2.118 2.995 2.118 | 4.527 8.120 6.741 | - 11.116 - | 6.92% | ||
| (110)TiN‖(110)Fe3C | 2.995 3.668 2.118 | 8.448 9.585 4.527 | - 26.551 - | 5.16% | ||
| (111)TiN‖(111)Fe3C | 2.995 2.995 2.955 | 8.448 8.120 6.813 | 3.227 8.258 - | 9.25% | ||
| Fe3C-Fe7C3 | [hkl]s | [hkl]n | θ (°) | Disregistry | ||
| (110)Fe3C‖(110)Fe7C3 | [001] | [001] | 4.527 9.585 8.448 | 11.913 14.492 8.251 | - 27.107 - | 11.38% |
| (100)Fe3C‖(100)Fe7C3 | [001] [011] [010] | [001] [011] [010] | 4.527 8.120 6.741 | 11.913 13.763 6.892 | - 26.068 - | - 7.40% - |
| (111)FeC3‖(111)Fe7C3 | 8.120 8.448 6.813 | 13.763 12.748 8.251 | 2.983 15.488 - | 21.0% | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Q.; Wang, G.; Yuan, X.; Wang, Q.; Sridhar, S. Complex Precipitates of TiN-MCx in GCr15 Bearing Steel. Metals 2019, 9, 641. https://doi.org/10.3390/met9060641
Tian Q, Wang G, Yuan X, Wang Q, Sridhar S. Complex Precipitates of TiN-MCx in GCr15 Bearing Steel. Metals. 2019; 9(6):641. https://doi.org/10.3390/met9060641
Chicago/Turabian StyleTian, Qianren, Guocheng Wang, Xinghu Yuan, Qi Wang, and Seetharaman Sridhar. 2019. "Complex Precipitates of TiN-MCx in GCr15 Bearing Steel" Metals 9, no. 6: 641. https://doi.org/10.3390/met9060641
APA StyleTian, Q., Wang, G., Yuan, X., Wang, Q., & Sridhar, S. (2019). Complex Precipitates of TiN-MCx in GCr15 Bearing Steel. Metals, 9(6), 641. https://doi.org/10.3390/met9060641