Medicinal Plants and Their Bacterial Microbiota: A Review on Antimicrobial Compounds Production for Plant and Human Health
Abstract
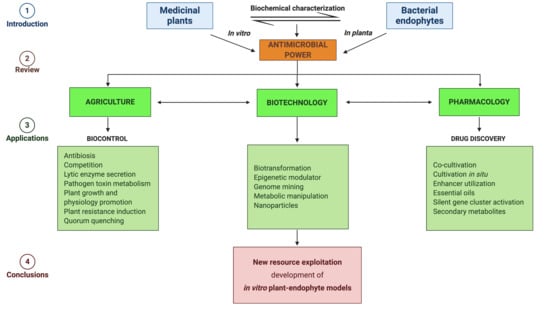
:1. Introduction to Medicinal Plants
1.1. MPs and Antimicrobial Power
1.2. MPs Bacterial Endophytes
Experimental Approaches for the Study of MP-Bacteria Interaction
2. Antimicrobial Power of the MPs Bacterial Microbiota
2.1. Studies on Antimicrobial Activity of MP Bacterial Endophytes
2.2. Studies on the Role of MP-Endophyte Interaction in the Production of Bioactive Molecules: Focus on Antimicrobial Compounds
3. Biotechnological and Therapeutical Applications
3.1. Biological Control of Plant Pathogens
3.2. Discover New Molecules to Fight Human MDR Pathogen
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Firenzuoli, F.; Gori, L. Herbal medicine today: Clinical and research issues. Evid. Based Complement. Altern. Med. 2007, 4, 37–40. [Google Scholar] [CrossRef]
- Williamson, E.M. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Firenzuoli, F.; Gori, L.; Neri, D. Clinical phytotherapy: Opportunities and problematics. Ann. Ist. Super. Sanita 2005, 41, 27–33. [Google Scholar] [PubMed]
- Kharwar, R.N.; Sharma, V.K.; Mishra, A.; Kumar, J.; Singh, D.K.; Verma, S.K.; Gond, S.K.; Kumar, A.; Kaushik, N.; Revuru, B.; et al. Harnessing the phytotherapeutic treasure troves of the ancient medicinal plant azadirachta indica (Neem) and associated endophytic microorganisms. Planta Med. 2020, 86, 906–940. [Google Scholar]
- Jalaluddin, M.; Rajasekaran, U.B.; Paul, S.; Dhanya, R.S.; Sudeep, C.B.; Adarsh, V.J. Comparative evaluation of neem mouthwash on plaque and gingivitis: A double-blind crossover study. J. Contemp. Dent. Pract. 2017, 18, 567–571. [Google Scholar] [CrossRef]
- European Medicines Agency Recommends New Malaria Treatment for Approval. Available online: https://www.ema.europa.eu/en/news/european-medicines-agency-recommends-new-malaria-treatment-approval (accessed on 6 January 2021).
- Arora, M.; Saxena, P.; Choudhary, D.K.; Abdin, M.Z.; Varma, A. Dual symbiosis between Piriformospora indica and Azotobacter chroococcum enhances the artemisinin content in Artemisia annua L. World J. Microbiol. Biotechnol. 2016, 32, 1–10. [Google Scholar] [CrossRef]
- Li, J.; Zhao, G.Z.; Varma, A.; Qin, S.; Xiong, Z.; Huang, H.Y.; Zhu, W.Y.; Zhao, L.X.; Xu, L.H.; Zhang, S.; et al. An endophytic Pseudonocardia species induces the production of Artemisinin in Artemisia annua. PLoS ONE 2012, 7, e51410. [Google Scholar] [CrossRef]
- Nardoni, S.; Mugnaini, L.; Pistelli, L.; Leonardi, M.; Sanna, V.; Perrucci, S.; Pisseri, F.; Mancianti, F. Clinical and mycological evaluation of an herbal antifungal formulation in canine Malassezia dermatitis. J. Mycol. Med. 2014, 24, 234–240. [Google Scholar] [CrossRef]
- Perić, A.; Gaćeša, D.; Barać, A.; Sotirović, J.; Perić, A.V. Herbal Drug EPs 7630 versus Amoxicillin in Patients with Uncomplicated Acute Bacterial Rhinosinusitis: A Randomized, Open-Label Study. Ann. Otol. Rhinol. Laryngol. 2020, 129, 969–976. [Google Scholar] [CrossRef]
- Xie, Q.; Johnson, B.R.; Wenckus, C.S.; Fayad, M.I.; Wu, C.D. Efficacy of berberine, an antimicrobial plant alkaloid, as an endodontic irrigant against a mixed-culture biofilm in an in vitro tooth model. J. Endod. 2012, 38, 1114–1117. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X.; Tian, S.; Chen, L.; Yuan, J. li Bioinformatics analysis of endophytic bacteria related to berberine in the Chinese medicinal plant Coptis teeta Wall. 3 Biotech 2020, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Maggini, V.; De Leo, M.; Mengoni, A.; Gallo, E.R.; Miceli, E.; Reidel, R.V.B.; Biffi, S.; Pistelli, L.; Fani, R.; Firenzuoli, F.; et al. Plant-endophytes interaction influences the secondary metabolism in Echinacea purpurea (L.) Moench: An in vitro model. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Maggini, V.; De Leo, M.; Granchi, C.; Tuccinardi, T.; Mengoni, A.; Gallo, E.R.; Biffi, S.; Fani, R.; Pistelli, L.; Firenzuoli, F.; et al. The influence of Echinacea purpurea leaf microbiota on chicoric acid level. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Naby Awad, O.G. Echinacea can help with Azithromycin in prevention of recurrent tonsillitis in children. Am. J. Otolaryngol. Head Neck Med. Surg. 2020, 41, 102344. [Google Scholar] [CrossRef] [PubMed]
- Isbaniah, F.; Wiyono, W.H.; Yunus, F.; Setiawati, A.; Totzke, U.; Verbruggen, M.A. Echinacea purpurea along with zinc, selenium and vitamin C to alleviate exacerbations of chronic obstructive pulmonary disease: Results from a randomized controlled trial. J. Clin. Pharm. Ther. 2011, 36, 568–576. [Google Scholar] [CrossRef]
- Wicaksono, W.A.; Jones, E.E.; Monk, J.; Ridgway, H.J. The Bacterial Signature of Leptospermum scoparium (Mānuka) Reveals Core and Accessory Communities with Bioactive Properties. PLoS ONE 2016, 11, e0163717. [Google Scholar] [CrossRef] [Green Version]
- Ooi, M.L.; Jothin, A.; Bennett, C.; Ooi, E.H.; Vreugde, S.; Psaltis, A.J.; Wormald, P.J. Manuka honey sinus irrigations in recalcitrant chronic rhinosinusitis: Phase 1 randomized, single-blinded, placebo-controlled trial. Int. Forum Allergy Rhinol. 2019, 9, 1470–1477. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Simon, J.C.; Marchesi, J.R.; Mougel, C.; Selosse, M.A. Host-microbiota interactions: From holobiont theory to analysis. Microbiome 2019, 7, 5. [Google Scholar] [CrossRef]
- Sessitsch, A.; Pfaffenbichler, N.; Mitter, B. Microbiome Applications from Lab to Field: Facing Complexity. Trends Plant Sci. 2019, 24, 194–198. [Google Scholar] [CrossRef]
- Mengoni, A.; Pini, F.; Huang, L.N.; Shu, W.S.; Bazzicalupo, M. Plant-by-plant variations of bacterial communities associated with leaves of the nickel hyperaccumulator Alyssum bertolonii desv. Microb. Ecol. 2009, 58, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Mengoni, A.; Schat, H.; Vangronsveld, J. Plants as extreme environments? Ni-resistant bacteria and Ni-hyperaccumulators of serpentine flora. Plant Soil 2010, 331, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Thijs, S.; Sillen, W.; Rineau, F.; Weyens, N.; Vangronsveld, J. Towards an enhanced understanding of plant-microbiome interactions to improve phytoremediation: Engineering the metaorganism. Front. Microbiol. 2016, 7, 341. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.B.; Vogel, C.; Bai, Y.; Vorholt, J.A. The Plant Microbiota: Systems-Level Insights and Perspectives. Annu. Rev. Genet. 2016, 50, 211–234. [Google Scholar] [CrossRef] [Green Version]
- Green, J.L.; Bohannan, B.J.M.; Whitaker, R.J. Microbial biogeography: From taxonomy to traits. Science 2008, 320, 1039–1043. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Cui, Y.; Li, H.; Kuang, A.; Li, X.; Wei, Y.; Xiuling, J.I. Diversity and composition of rhizospheric soil and root endogenous bacteria in Panax notoginseng during continuous cropping practices. J. Basic Microbiol. 2017, 57, 337–344. [Google Scholar] [CrossRef]
- Köberl, M.; Schmidt, R.; Ramadan, E.M.; Bauer, R.; Berg, G. The microbiome of medicinal plants: Diversity and importance for plant growth, quality, and health. Front. Microbiol. 2013, 4, 400. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, R.J.; Henson, J.; Van Volkenburgh, E.; Hoy, M.; Wright, L.; Beckwith, F.; Kim, Y.O.; Redman, R.S. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2008, 2, 404–416. [Google Scholar] [CrossRef] [Green Version]
- Wardle, D.A. The influence of biotic interactions on soil biodiversity. Ecol. Lett. 2006, 9, 870–886. [Google Scholar] [CrossRef]
- Mengoni, A.; Maida, I.; Chiellini, C.; Emiliani, G.; Mocali, S.; Fabiani, A.; Fondi, M.; Firenzuoli, F.; Fani, R. Antibiotic resistance differentiates Echinacea purpurea endophytic bacterial communities with respect to plant organs. Res. Microbiol. 2014, 165, 686–694. [Google Scholar] [CrossRef]
- Pontonio, E.; Di Cagno, R.; Tarraf, W.; Filannino, P.; De Mastro, G.; Gobbetti, M. Dynamic and Assembly of Epiphyte and Endophyte Lactic Acid Bacteria During the Life Cycle of Origanum vulgare L. Front. Microbiol. 2018, 9, 1372. [Google Scholar] [CrossRef] [PubMed]
- Checcucci, A.; Maida, I.; Bacci, G.; Ninno, C.; Bilia, A.R.; Biffi, S.; Firenzuoli, F.; Flamini, G.; Fani, R.; Mengoni, A. Is the plant-associated microbiota of Thymus spp. adapted to plant essential oil? Res. Microbiol. 2017, 168, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Brader, G.; Compant, S.; Mitter, B.; Trognitz, F.; Sessitsch, A. Metabolic potential of endophytic bacteria. Curr. Opin. Biotechnol. 2014, 27, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Kusari, P.; Kusari, S.; Eckelmann, D.; Zühlke, S.; Kayser, O.; Spiteller, M. Cross-species biosynthesis of maytansine in Maytenus serrata. RSC Adv. 2016, 6, 10011–10016. [Google Scholar] [CrossRef] [Green Version]
- Maida, I.; Chiellini, C.; Mengoni, A.; Bosi, E.; Firenzuoli, F.; Fondi, M.; Fani, R. Antagonistic interactions between endophytic cultivable bacterial communities isolated from the medicinal plant Echinacea purpurea. Environ. Microbiol. 2016, 18, 2357–2365. [Google Scholar] [CrossRef]
- Castronovo, L.M.; Calonico, C.; Ascrizzi, R.; Del Duca, S.; Delfino, V.; Chioccioli, S.; Vassallo, A.; Strozza, I.; De Leo, M.; Biffi, S.; et al. The Cultivable Bacterial Microbiota Associated to the Medicinal Plant Origanum vulgare L.: From Antibiotic Resistance to Growth-Inhibitory Properties. Front. Microbiol. 2020, 11, 862. [Google Scholar] [CrossRef]
- Chiellini, C.; Maida, I.; Maggini, V.; Bosi, E.; Mocali, S.; Emiliani, G.; Perrin, E.; Firenzuoli, F.; Mengoni, A.; Fani, R. Preliminary data on antibacterial activity of Echinacea purpurea-associated bacterial communities against Burkholderia cepacia complex strains, opportunistic pathogens of Cystic Fibrosis patients. Microbiol. Res. 2017, 196, 34–43. [Google Scholar] [CrossRef]
- Presta, L.; Bosi, E.; Fondi, M.; Maida, I.; Perrin, E.; Miceli, E.; Maggini, V.; Bogani, P.; Firenzuoli, F.; Di Pilato, V.; et al. Phenotypic and genomic characterization of the antimicrobial producer Rheinheimera sp. EpRS3 isolated from the medicinal plant Echinacea purpurea: Insights into its biotechnological relevance. Res. Microbiol. 2017, 168, 293–305. [Google Scholar] [CrossRef]
- Nemr, R.A.; Khalil, M.; Sarhan, M.S.; Abbas, M.; Elsawey, H.; Youssef, H.H.; Hamza, M.A.; Morsi, A.T.; El-Tahan, M.; Fayez, M.; et al. “In situ similis” Culturing of Plant Microbiota: A Novel Simulated Environmental Method Based on Plant Leaf Blades as Nutritional Pads. Front. Microbiol. 2020, 11, 454. [Google Scholar] [CrossRef] [Green Version]
- Harrison, J.G.; Griffin, E.A. The diversity and distribution of endophytes across biomes, plant phylogeny and host tissues: How far have we come and where do we go from here? Environ. Microbiol. 2020, 22, 2107–2123. [Google Scholar] [CrossRef] [Green Version]
- Akinsanya, M.A.; Goh, J.K.; Lim, S.P.; Ting, A.S.Y. Metagenomics study of endophytic bacteria in Aloe vera using next-generation technology. Genom. Data 2015, 6, 159–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emiliani, G.; Mengoni, A.; Maida, I.; Perrin, E.; Chiellini, C.; Fondi, M.; Gallo, E.; Gori, L.; Maggini, V.; Vannacci, A.; et al. Linking bacterial endophytic communities to essential oils: Clues from Lavandula angustifolia mill. Evid. Based Complement. Altern. Med. 2014, 2014, 650905. [Google Scholar] [CrossRef] [PubMed]
- Chiellini, C.; Maida, I.; Emiliani, G.; Mengoni, A.; Mocali, S.; Fabiani, A.; Biffi, S.; Maggini, V.; Gori, L.; Vannacci, A.; et al. Endophytic and rhizospheric bacterial communities isolated from the medicinal plants echinacea purpurea and echinacea angustifolia. Int. Microbiol. 2015, 17, 165–174. [Google Scholar] [CrossRef]
- Strobel, G.A. Endophytes as sources of bioactive products. Microbes Infect. 2003, 5, 535–544. [Google Scholar] [CrossRef]
- Jasim, B.; Sreelakshmi, S.; Mathew, J.; Radhakrishnan, E.K. Identification of endophytic Bacillus mojavensis with highly specialized broad spectrum antibacterial activity. 3 Biotech 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Yasmin, S.; Ghosh, S.; Bhattacharya, S.; Banerjee, D. Anti-Infective Metabolites of a Newly Isolated Bacillus thuringiensis KL1 Associated with Kalmegh (Andrographis paniculata Nees.), a Traditional Medicinal Herb. Microbiol. Insights 2016, 9, MBI.S33394. [Google Scholar] [CrossRef] [PubMed]
- Castillo, U.F.; Strobel, G.A.; Ford, E.J.; Hess, W.M.; Porter, H.; Jensen, J.B.; Albert, H.; Robison, R.; Condron, M.A.M.; Teplow, D.B.; et al. Munumbicins, wide-spectrum antibiotics produced by Streptomyces NRRL 30562, endophytic on Kennedia nigriscans. Microbiology 2002, 148, 2675–2685. [Google Scholar] [CrossRef] [Green Version]
- Conti, R.; Chagas, F.O.; Caraballo-Rodriguez, A.M.; da Paixão Melo, W.G.; do Nascimento, A.M.; Cavalcanti, B.C.; de Moraes, M.O.; Pessoa, C.; Costa-Lotufo, L.V.; Krogh, R.; et al. Endophytic Actinobacteria from the Brazilian Medicinal Plant Lychnophora ericoides Mart. and the Biological Potential of Their Secondary Metabolites. Chem. Biodivers. 2016, 13, 727–736. [Google Scholar] [CrossRef]
- Taechowisan, T.; Chanaphat, S.; Ruensamran, W.; Phutdhawong, W.S. Antifungal activity of 3-methylcarbazoles from Streptomyces sp. LJK109; an endophyte in Alpinia galanga. J. Appl. Pharm. Sci. 2012, 2, 255820. [Google Scholar]
- Ludwig-Müller, J. Plants and endophytes: Equal partners in secondary metabolite production? Biotechnol. Lett. 2015, 37, 1325–1334. [Google Scholar] [CrossRef]
- Li, J.; Zhao, G.Z.; Huang, H.Y.; Qin, S.; Zhu, W.Y.; Zhao, L.X.; Xu, L.H.; Zhang, S.; Li, W.J.; Strobel, G. Isolation and characterization of culturable endophytic actinobacteria associated with Artemisia annua L. Antonie Van Leeuwenhoekint. J. Gen. Mol. Microbiol. 2012, 101, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.S.; Singh, S.; Babu, C.S.V.; Shanker, K.; Srivastava, N.K.; Kalra, A. Endophytes of opium poppy differentially modulate host plant productivity and genes for the biosynthetic pathway of benzylisoquinoline alkaloids. Planta 2016, 243, 1097–1114. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.S.; Singh, S.; Pandey, H.; Srivastava, M.; Ray, T.; Soni, S.; Pandey, A.; Shanker, K.; Babu, C.S.V.; Banerjee, S.; et al. Endophytes of Withania somnifera modulate in planta content and the site of withanolide biosynthesis. Sci. Rep. 2018, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Awasthi, A.; Mall, M.; Shukla, A.K.; Srinivas, K.V.N.S.; Syamasundar, K.V.; Kalra, A. Bacterial endophyte-mediated enhancement of in planta content of key terpenoid indole alkaloids and growth parameters of Catharanthus roseus. Ind. Crop. Prod. 2013, 43, 306–310. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, J.; Li, Y.; Wen, J.; Wang, R. Bacterial endophytes from Lycoris radiata promote the accumulation of Amaryllidaceae alkaloids. Microbiol. Res. 2020, 239, 126501. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Wirth, S.; Behrendt, U.; Ahmad, P.; Berg, G. Antimicrobial Activity of Medicinal Plants Correlates with the Proportion of Antagonistic Endophytes. Front. Microbiol. 2017, 8, 199. [Google Scholar] [CrossRef] [Green Version]
- Passari, A.K.; Leo, V.V.; Singh, G.; Samanta, L.; Ram, H.; Siddaiah, C.N.; Hashem, A.; Al-Arjani, A.B.F.; Alqarawi, A.A.; Abd_Allah, E.F.; et al. In vivo studies of inoculated plants and in vitro studies utilizing methanolic extracts of endophytic Streptomyces sp. Strain dbt34 obtained from Mirabilis jalapa L. exhibit ros-scavenging and other bioactive properties. Int. J. Mol. Sci. 2020, 21, 7364. [Google Scholar] [CrossRef]
- Mishra, A.; Singh, S.P.; Mahfooz, S.; Bhattacharya, A.; Mishra, N.; Shirke, P.A.; Nautiyal, C.S. Bacterial endophytes modulates the withanolide biosynthetic pathway and physiological performance in Withania somnifera under biotic stress. Microbiol. Res. 2018, 212, 17–28. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Li, X.; Zhao, D.; Deng-Wang, M.Y.; Dai, C.C. Reactive oxygen species and hormone signaling cascades in endophytic bacterium induced essential oil accumulation in Atractylodes lancea. Planta 2016, 244, 699–712. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Sun, K.; Chen, F.; Yuan, J.; Li, X.; Dai, C.C. Endophytic Pseudomonas induces metabolic flux changes that enhance medicinal sesquiterpenoid accumulation in Atractylodes lancea. Plant Physiol. Biochem. 2018, 130, 473–481. [Google Scholar] [CrossRef]
- Qin, S.; Feng, W.W.; Zhang, Y.J.; Wang, T.T.; Xiong, Y.W.; Xing, K. Diversity of bacterial microbiota of coastal halophyte Limonium sinense and amelioration of salinity stress damage by symbiotic plant growthpromoting actinobacterium Glutamicibacter halophytocola KLBMP 5180. Appl. Environ. Microbiol. 2018, 84, 1533–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devi, K.A.; Pandey, G.; Rawat, A.K.S.; Sharma, G.D.; Pandey, P. The Endophytic Symbiont—Pseudomonas aeruginosa Stimulates the Antioxidant Activity and Growth of Achyranthes aspera L. Front. Microbiol. 2017, 8, 1897. [Google Scholar] [CrossRef] [Green Version]
- Purushotham, N.; Jones, E.; Monk, J.; Ridgway, H. Community Structure of Endophytic Actinobacteria in a New Zealand Native Medicinal Plant Pseudowintera colorata (Horopito) and Their Influence on Plant Growth. Microb. Ecol. 2018, 76, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Ulloa-Muñoz, R.; Olivera-Gonzales, P.; Castañeda-Barreto, A.; Villena, G.K.; Tamariz-Angeles, C. Diversity of endophytic plant-growth microorganisms from Gentianella weberbaueri and Valeriana pycnantha, highland Peruvian medicinal plants. Microbiol. Res. 2020, 233. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.S.A.; El-Sayed, M.T.; Rady, A.M.; Zein, N.; Enan, G.; Shindia, A.; El-Hefnawy, S.; Sitohy, M.; Sitohy, B. Exploiting the Biosynthetic Potency of Taxol from Fungal Endophytes of Conifers Plants; Genome Mining and Metabolic Manipulation. Molecules 2020, 25, 3000. [Google Scholar] [CrossRef]
- Christina, A.; Christapher, V.; Bhore, S. Endophytic bacteria as a source of novel antibiotics: An overview. Pharmacogn. Rev. 2013, 7, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Kumar, A.; Singh, R.; Pandey, K.D. Endophytic bacteria: A new source of bioactive compounds. 3 Biotech 2017, 7. [Google Scholar] [CrossRef]
- Quinn, G.A.; Banat, A.M.; Abdelhameed, A.M.; Banat, I.M. Streptomyces from traditional medicine: Sources of new innovations in antibiotic discovery. J. Med. Microbiol. 2020, 69, 1040–1048. [Google Scholar] [CrossRef]
- Nagpal, S.; Sharma, P.; Sirari, A.; Gupta, R.K. Coordination of Mesorhizobium sp. and endophytic bacteria as elicitor of biocontrol against Fusarium wilt in chickpea. Eur. J. Plant Pathol. 2020, 158, 143–161. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Giovanardi, D.; Stefani, E. Plant growth promoting and biocontrol activity of Streptomyces spp. as endophytes. Int. J. Mol. Sci. 2018, 19, 952. [Google Scholar] [CrossRef] [Green Version]
- Sundaramoorthy, S. Consortial Effect of Endophytic and Plant Growth Promoting Rhizobacteria for the Management of Early Blight of Tomato Incited by Alternaria Solani. J. Plant Pathol. Microbiol. 2012, 3, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Michel-Aceves, A.C.; Díaz-Nájera, J.F.; Ariza-Flores, R.; Otero-Sánchez, M.A.; Escobar-Martínez, R.; Avendaño-Arrazate, C.H. Control alternatives for damping-off in tomato seedling production. Phyton 2019, 88, 325–333. [Google Scholar] [CrossRef]
- Huang, X.; Ren, J.; Li, P.; Feng, S.; Dong, P.; Ren, M. Potential of microbial endophytes to enhance the resistance to postharvest diseases of fruit and vegetables. J. Sci. Food Agric. 2020, jsfa.10829. [Google Scholar] [CrossRef] [PubMed]
- Resti, Z.; Liswarni, Y.; Martinius, M. Endophytic Bacterial Consortia as Biological Control of Bacterial Leaf Blight and Plant Growth Promoter of Rice (Oryza sativa L.). J. Appl. Agric. Sci. Technol. 2020, 4, 134–145. [Google Scholar] [CrossRef]
- Pal, K.K.; McSpadden Gardener, B. Biological Control of Plant Pathogens. Plant Health Instr. 2006. [Google Scholar] [CrossRef] [Green Version]
- Lecomte, C.; Alabouvette, C.; Edel-Hermann, V.; Robert, F.; Steinberg, C. Biological control of ornamental plant diseases caused by Fusarium oxysporum: A review. Biol. Control 2016, 101, 17–30. [Google Scholar] [CrossRef]
- Eljounaidi, K.; Lee, S.K.; Bae, H. Bacterial endophytes as potential biocontrol agents of vascular wilt diseases—Review and future prospects. Biol. Control 2016, 103, 62–68. [Google Scholar] [CrossRef]
- Latz, M.A.C.; Jensen, B.; Collinge, D.B.; Jørgensen, H.J.L. Endophytic fungi as biocontrol agents: Elucidating mechanisms in disease suppression. Plant Ecol. Divers. 2018, 11, 555–567. [Google Scholar] [CrossRef] [Green Version]
- De Silva, N.I.; Brooks, S.; Lumyong, S.; Hyde, K.D. Use of endophytes as biocontrol agents. Fungal Biol. Rev. 2019, 33, 133–148. [Google Scholar] [CrossRef]
- Morales-Cedeño, L.R.; del Carmen Orozco-Mosqueda, M.; Loeza-Lara, P.D.; Parra-Cota, F.I.; de los Santos-Villalobos, S.; Santoyo, G. Plant growth-promoting bacterial endophytes as biocontrol agents of pre- and post-harvest diseases: Fundamentals, methods of application and future perspectives. Microbiol. Res. 2021, 242, 126612. [Google Scholar] [CrossRef]
- Santoyo, G.; del Orozco-Mosqueda, M.C.; Govindappa, M. Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: A review. Biocontrol Sci. Technol. 2012, 22, 855–872. [Google Scholar] [CrossRef]
- Ahmed, E.A.; Hassan, E.A.; Tobgy, K.M.K.E.; Ramadan, E.M. Evaluation of rhizobacteria of some medicinal plants for plant growth promotion and biological control. Ann. Agric. Sci. 2014, 59, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.K.; Pathak, R. Ecological Manipulations of Rhizobacteria for Curbing Medicinal Plant Diseases; Springer: Cham, Switzerland, 2015; pp. 217–230. [Google Scholar]
- Xie, H.; Feng, X.; Wang, M.; Wang, Y.; Kumar Awasthi, M.; Xu, P. Implications of endophytic microbiota in Camellia sinensis: A review on current understanding and future insights. Bioengineered 2020, 11, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Barloy, J.; Pelhate, J. Premieres observations phytopathologiques relatives aux cultures de chanvre en Anjou. Ann. Epiphyt. 1962, 13, 117–149. [Google Scholar]
- Doctor, B. Damping off. Sinsemilla Tips 1985, 5, 35–39. [Google Scholar]
- McPartland, J.M. Common names for diseases of Cannabis sativa L. Plant Dis. 1991, 75, 226–227. [Google Scholar]
- Snyder, W.C.; Hansen, H.N. The Species Concept in Fusarium. Am. J. Bot. 1940, 27, 64. [Google Scholar] [CrossRef]
- Taghinasab, M.; Jabaji, S. Cannabis Microbiome and the Role of Endophytes in Modulating the Production of Secondary Metabolites: An Overview. Microorganisms 2020, 8, 355. [Google Scholar] [CrossRef] [Green Version]
- Toyoda, H.; Utsumi, R. Method for the Prevention of Fusarium Diseases and Microorganisms Used for the Same. U.S. Patent 4,988,586, 29 January 1991. [Google Scholar]
- Simonetti, E.; Roberts, I.N.; Montecchia, M.S.; Gutierrez-Boem, F.H.; Gomez, F.M.; Ruiz, J.A. A novel Burkholderia ambifaria strain able to degrade the mycotoxin fusaric acid and to inhibit Fusarium spp. growth. Microbiol. Res. 2018, 206, 50–59. [Google Scholar] [CrossRef]
- Mauch, F.; Mauch-Mani, B.; Boller, T. Antifungal Hydrolases in Pea Tissue. Plant Physiol. 1988, 88, 936–942. [Google Scholar] [CrossRef] [Green Version]
- Sahu, P.K.; Singh, S.; Gupta, A.R.; Gupta, A.; Singh, U.B.; Manzar, N.; Bhowmik, A.; Singh, H.V.; Saxena, A.K. Endophytic bacilli from medicinal-aromatic perennial Holy basil (Ocimum tenuiflorum L.) modulate plant growth promotion and induced systemic resistance against Rhizoctonia solani in rice (Oryza sativa L.). Biol. Control 2020, 150, 104353. [Google Scholar] [CrossRef]
- Wicaksono, W.A.; Jones, E.E.; Casonato, S.; Monk, J.; Ridgway, H.J. Biological control of Pseudomonas syringae pv. actinidiae (Psa), the causal agent of bacterial canker of kiwifruit, using endophytic bacteria recovered from a medicinal plant. Biol. Control 2018, 116, 103–112. [Google Scholar] [CrossRef]
- Wicaksono, W.A.; Jones, E.E.; Sansom, C.E.; Perry, N.B.; Monk, J.; Black, A.; Ridgway, H.J. Indigenous bacteria enhance growth and modify essential oil content in Leptospermum scoparium (mānuka). N. Z. J. Bot. 2017, 55, 306–317. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [Green Version]
- Pandey, V.N.; Dubey, N.K. Antifungal potential of leaves and essential oils from higher plants against soil phytopathogens. Soil Biol. Biochem. 1994, 26, 1417–1421. [Google Scholar] [CrossRef]
- Kealey, C.; Creaven, C.A.; Murphy, C.D.; Brady, C.B. New approaches to antibiotic discovery. Biotechnol. Lett. 2017, 39, 805–817. [Google Scholar] [CrossRef]
- Martinez-Klimova, E.; Rodríguez-Peña, K.; Sánchez, S. Endophytes as sources of antibiotics. Biochem. Pharmacol. 2017, 134, 1–17. [Google Scholar] [CrossRef]
- Wu, C.; Zacchetti, B.; Ram, A.F.J.; Van Wezel, G.P.; Claessen, D.; Choi, Y.H. Expanding the chemical space for natural products by Aspergillus-Streptomyces co-cultivation and biotransformation. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Fukumoto, A.; Kim, Y.P.; Matsumoto, A.; Takahashi, Y.; Shiomi, K.; Tomoda, H.; Omura, S. Cyslabdan, a new potentiator of imipenem activity against methicillin-resistant Staphylococcus aureus, produced by Streptomyces sp. K04-0144: I. Taxonomy, fermentation, isolation and structural elucidation. J. Antibiot. 2008, 61, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Nichols, D.; Cahoon, N.; Trakhtenberg, E.M.; Pham, L.; Mehta, A.; Belanger, A.; Kanigan, T.; Lewis, K.; Epstein, S.S. Use of ichip for high-throughput in situ cultivation of “uncultivable microbial species”. Appl. Environ. Microbiol. 2010, 76, 2445–2450. [Google Scholar] [CrossRef] [Green Version]
- Yap, P.S.X.; Krishnan, T.; Yiap, B.C.; Hu, C.P.; Chan, K.G.; Lim, S.H.E. Membrane disruption and anti-quorum sensing effects of synergistic interaction between Lavandula angustifolia (lavender oil) in combination with antibiotic against plasmid-conferred multi-drug-resistant Escherichia coli. J. Appl. Microbiol. 2014, 116, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, T.F.; Vollú, R.E.; Jurelevicius, D.; Alviano, D.S.; Alviano, C.S.; Blank, A.F.; Seldin, L. Does the essential oil of Lippia sidoides Cham. (Pepper-rosmarin) affect its endophytic microbial community? Bmc Microbiol. 2013, 13, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrin, E.; Maggini, V.; Maida, I.; Gallo, E.; Lombardo, K.; Madarena, M.P.; Buroni, S.; Scoffone, V.C.; Firenzuoli, F.; Mengoni, A.; et al. Antimicrobial activity of six essential oils against Burkholderia cepacia complex: Insights into mechanism(s) of action. Future Microbiol. 2018, 13, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.H.; Van Nguyen, H.; Vu, T.H.N.; Chu-Ky, S.; Vu, T.T.; Hoang, H.; Quach, N.T.; Bui, T.L.; Chu, H.H.; Khieu, T.N.; et al. Characterization of endophytic Streptomyces griseorubens MPT42 and assessment of antimicrobial synergistic interactions of its extract and essential oil from host plant Litsea cubeba. Antibiotics 2019, 8, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, D.; Chatterjee, S. Antibacterial Activity of Green Synthesized Silver Nanoparticles Using Vasaka (Justicia adhatoda L.) Leaf Extract. Indian J. Microbiol. 2015, 55, 163–167. [Google Scholar] [CrossRef] [Green Version]
- Thirumagal, N.; Jeyakumari, A.P. Structural, Optical and Antibacterial Properties of Green Synthesized Silver Nanoparticles (AgNPs) Using Justicia adhatoda L. Leaf Extract. J. Clust. Sci. 2020, 31, 487–497. [Google Scholar] [CrossRef]
- Sunkar, S.; Nachiyar, C.V. Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymus. Asian Pac. J. Trop. Biomed. 2012, 2, 953–959. [Google Scholar] [CrossRef] [Green Version]
- Monowar, T.; Rahman, S.; Bhore, S.J.; Raju, G.; Sathasivam, K.V. Silver Nanoparticles Synthesized by Using the Endophytic Bacterium Pantoea ananatis are Promising Antimicrobial Agents against Multidrug Resistant Bacteria. Molecules 2018, 23, 3220. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Wang, J.; Huang, Y.; Chen, H.; Li, Y.; Zhong, L.; Chen, Y.; Chen, S.; Wang, J.; Kang, J.; et al. Anti-mycobacterial activity of marine fungus-derived 4-deoxybostrycin and nigrosporin. Molecules 2013, 18, 1728–1740. [Google Scholar] [CrossRef] [Green Version]
- Kumar, J.; Sharma, V.K.; Singh, D.K.; Mishra, A.; Gond, S.K.; Verma, S.K.; Kumar, A.; Kharwar, R.N. Epigenetic Activation of Antibacterial Property of an Endophytic Streptomyces coelicolor Strain AZRA 37 and Identification of the Induced Protein Using MALDI TOF MS/MS. PLoS ONE 2016, 11, e0147876. [Google Scholar] [CrossRef] [Green Version]
- Maggini, V.; Mengoni, A.; Bogani, P.; Firenzuoli, F.; Fani, R. Promoting Model Systems of Microbiota–Medicinal Plant Interactions. Trends Plant Sci. 2020, 25, 223–225. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castronovo, L.M.; Vassallo, A.; Mengoni, A.; Miceli, E.; Bogani, P.; Firenzuoli, F.; Fani, R.; Maggini, V. Medicinal Plants and Their Bacterial Microbiota: A Review on Antimicrobial Compounds Production for Plant and Human Health. Pathogens 2021, 10, 106. https://doi.org/10.3390/pathogens10020106
Castronovo LM, Vassallo A, Mengoni A, Miceli E, Bogani P, Firenzuoli F, Fani R, Maggini V. Medicinal Plants and Their Bacterial Microbiota: A Review on Antimicrobial Compounds Production for Plant and Human Health. Pathogens. 2021; 10(2):106. https://doi.org/10.3390/pathogens10020106
Chicago/Turabian StyleCastronovo, Lara Mitia, Alberto Vassallo, Alessio Mengoni, Elisangela Miceli, Patrizia Bogani, Fabio Firenzuoli, Renato Fani, and Valentina Maggini. 2021. "Medicinal Plants and Their Bacterial Microbiota: A Review on Antimicrobial Compounds Production for Plant and Human Health" Pathogens 10, no. 2: 106. https://doi.org/10.3390/pathogens10020106
APA StyleCastronovo, L. M., Vassallo, A., Mengoni, A., Miceli, E., Bogani, P., Firenzuoli, F., Fani, R., & Maggini, V. (2021). Medicinal Plants and Their Bacterial Microbiota: A Review on Antimicrobial Compounds Production for Plant and Human Health. Pathogens, 10(2), 106. https://doi.org/10.3390/pathogens10020106