The Synthesis and Evaluation of Multivalent Glycopeptoids as Inhibitors of the Adhesion of Candida albicans
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Synthesis
2.2. Biological Evaluation
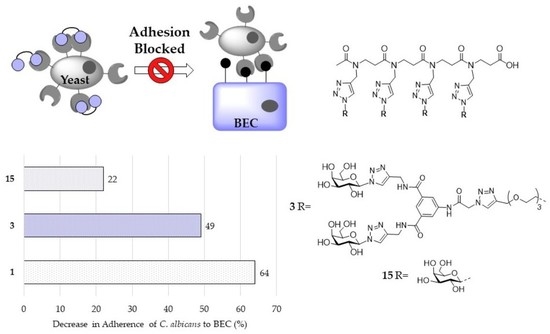
2.2.1. Exclusion Assay
2.2.2. Competition Assay
3. Materials and Methods
3.1. Chemistry
3.1.1. General Methods
3.1.2. Synthetic Procedures
Synthesis of N,N’-di-(2,3,4,6-tetra-O-acetyl-β-d-galactopyranosyl-1,2,3-triazol-4-ylmethylamide)-N”-(2-bromoacetamido)-5-aminobenzene-1,3-dicarboxamide (7)
Synthesis of N,N’-di-(2,3,4,6-tetra-O-acetyl-β-d-galactopyranosyl-1,2,3-triazol-4-ylmethyl amide)-N”-(2-azidoacetamido)-5-aminobenzene-1,3-dicarboxamide (8)
Synthesis of 2-[2-(2-Propargyloxyethoxy)ethoxy]ethanol (9)
Synthesis of 2-(2-(2-Propargyloxyethoxy)ethoxy)ethyl-4-methylbenzenesulfonate (10)
Synthesis of N, N’-di-(2,3,4,6-tetra-O-acetyl-β-d-galactopyranosyl-1,2,3-triazol-4-ylmethyl amide)-N”-(2-4-((2-(2-(2-(4-methylbenzenesulfonate)ethoxy)ethoxy)ethoxy) methyl)-1H-1,2,3-triazol-1-yl)acetamido)-5-aminobenzene-1,3-dicarboxamide (11)
Synthesis of N,N’-di-(2,3,4,6-tetra-O-acetyl-β-d-galactopyranosyl-1,2,3-triazol-4-ylmethylamide)-N”-(2-4-((2-(2-(2-azidoethoxy)ethoxy)ethoxy)methyl)-1H-1,2,3-triazol-1-yl)acetamido)-5-aminobenzene-1,3-dicarboxamide (12)
Synthesis of tert-butyl-(4,8,12,16-tetra-aza)(5,9,13,17-tetra-oxo)(4,8,12,16-tetra-N-propargyl)octadeconoate (2)
Synthesis of Acetylated Tetravalent β-Peptoid Glycocluster (13)
Synthesis of Tetravalent β-Peptoid Glycocluster (3)
Synthesis of 2,6,10,14-tetraoxo-3,7,11,15-tetrakis((1-(2,3,4,6-tetra-O-acetyl-β-d-galactopyranosyl -1H-1,2,3-triazol-4-yl)methyl)-3,7,11,15-tetraazaoctadecan-18-oic acid (14)
Synthesis of 2,6,10,14-tetraoxo-3,7,11,15-tetrakis((1-(β-d-galactopyranosyl-1H-1,2,3-triazol-4-yl)methyl)-3,7,11,15-tetraazaoctadecan-18-oic acid (15)
3.2. Biological Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varki, A. Biological roles of glycans. Glycobiology 2012, 27, 3–49. [Google Scholar] [CrossRef] [Green Version]
- Cozens, D.; Read, R.C. Anti-adhesion methods as novel therapeutics for bacterial infections. Expert Rev. Anti-Infect. Ther. 2012, 10, 1457–1468. [Google Scholar] [CrossRef]
- Linman, M.J.; Taylor, J.D.; Yu, H.; Chen, X.; Cheng, Q. Surface Plasmon Resonance Study of Protein−Carbohydrate Interactions Using Biotinylated Sialosides. Anal. Chem. 2008, 80, 4007–4013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimick, S.M.; Powell, S.C.; McMahon, S.A.; Moothoo, D.N.; Naismith, J.H.; Toone, E.J. On the Meaning of Affinity: Cluster Glycoside Effects and Concanavalin, A.J. Am. Chem. Soc. 1999, 121, 10286–10296. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lee, R.T. Carbohydrate-Protein Interactions: Basis of Glycobiology. Acc. Chem. Res. 1995, 28, 321–327. [Google Scholar] [CrossRef]
- Boukerb, A.M.; Rousset, A.; Galanos, N.; Méar, J.-B.; Thépaut, M.; Grandjean, T.; Gillon, E.; Cecioni, S.; Abderrahmen, C.; Faure, K.; et al. Antiadhesive Properties of Glycoclusters against Pseudomonas aeruginosa Lung Infection. J. Med. Chem. 2014, 57, 10275–10289. [Google Scholar] [CrossRef] [PubMed]
- Chabre, Y.M.; Roy, R. Multivalent glycoconjugate syntheses and applications using aromatic scaffolds. Chem. Soc. Rev. 2013, 42, 4657–4708. [Google Scholar] [CrossRef]
- Martínez, Á.; Ortiz Mellet, C.; García Fernández, J.M. Cyclodextrin-based multivalent glycodisplays: Covalent and supramolecular conjugates to assess carbohydrate–protein interactions. Chem. Soc. Rev. 2013, 42, 4746–4773. [Google Scholar] [CrossRef] [Green Version]
- Galan, M.C.; Dumy, P.; Renaudet, O. Multivalent glyco(cyclo)peptides. Chem. Soc. Rev. 2013, 42, 4599–4612. [Google Scholar] [CrossRef]
- Miura, Y. Design and synthesis of well-defined glycopolymers for the control of biological functionalities. Polym. J. 2012, 44, 679–689. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, G.; Becer, C.R. Glyconanoparticles and their interactions with lectins. Polym. Chem. 2015, 6, 5503–5514. [Google Scholar] [CrossRef] [Green Version]
- Hill, S.; Galan, M.C. Fluorescent carbon dots from mono- and polysaccharides: Synthesis, properties and applications. Beilstein J. Org. Chem. 2017, 13, 675–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardi, A.; Jimenez-Barbero, J.; Casnati, A.; De Castro, C.; Darbre, T.; Fieschi, F.; Finne, J.; Funken, H.; Jaeger, K.-E.; Lahmann, M.; et al. Multivalent glycoconjugates as anti-pathogenic agents. Chem. Soc. Rev. 2013, 42, 4709–4727. [Google Scholar] [CrossRef]
- Pieters, R.J. Maximising multivalency effects in protein–carbohydrate interactions. Org. Biomol. Chem. 2009, 7, 2013–2025. [Google Scholar] [CrossRef]
- Pifferi, C.; Goyard, D.; Gillon, E.; Imberty, A.; Renaudet, O. Synthesis of Mannosylated Glycodendrimers and Evaluation against BC2L-A Lectin from Burkholderia cenocepacia. ChemPlusChem 2017, 82, 390–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arsiwala, A.; Castro, A.; Frey, S.; Stathos, M.; Kane, R.S. Designing Multivalent Ligands to Control Biological Interactions: From Vaccines and Cellular Effectors to Targeted Drug Delivery. Chem. Asian J. 2019, 14, 244–255. [Google Scholar] [CrossRef] [Green Version]
- Brissonnet, Y.; Araoz, R.; Sousa, R.; Percevault, L.; Brument, S.; Deniaud, D.; Servant, D.; Le Questel, J.Y.; Lebreton, J.; Gouin, S.G. Di- and heptavalent nicotinic analogues to interfere with α7 nicotinic acetylcholine receptors. Bioorg. Med. Chem. 2019, 27, 700–707. [Google Scholar] [CrossRef]
- González-Cuesta, M.; Ortiz-Mellet, C.; García Fernández, J.M. Carbohydrate supramolecular chemistry: Beyond the multivalent effect. Chem. Commun. 2020, 56, 5207–5222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalopin, T.; Brissonnet, Y.; Sivignon, A.; Deniaud, D.; Cremet, L.; Barnich, N.; Bouckaert, J.; Gouin, S. Inhibition profiles of mono- and polyvalent FimH antag onists against 10 different Escherichia coli strains. Org. Biomol. Chem. 2015, 13, 11369–11375. [Google Scholar] [CrossRef]
- Lehot, V.; Brissonnet, Y.; Dussouy, C.; Brument, S.; Cabanettes, A.; Gillon, E.; Deniaud, D.; Varrot, A.; Pape, P.L. Multivalent fucosides with nanomolar affinity for the Aspergillus fumigatus lectin FleA prevent spore adhesion to Pneumocytes. Chem. Eur. J. 2018, 24, 19243–19249. [Google Scholar] [CrossRef]
- Revie, N.M.; Iyer, K.R.; Robbins, R.; Cowen, L.E. Antifungal drug resistance: Evolution, mechanisms and impact. Curr. Opin. Microbiol. 2018, 45, 70–76. [Google Scholar] [CrossRef]
- Ciurea, C.N.; Kosovski, I.B.; Mare, A.D.; Toma, F.; Pintea-Simon, I.A.; Man, A. Candida and Candidiasis-Opportunism Versus Pathogenicity: A Review of the Virulence Traits. Microorganisms 2020, 8, 857. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.; Govern, M.M.; Abbey, L.; Gilroy, A.; Mullins, S.; Howell, S.; Kavanagh, K.; Velasco-Torrijos, T. Inhibition of adherence of the yeast Candida albicans to buccal epithelial cells by synthetic aromatic glycoconjugates. Eur. J. Med. Chem. 2018, 160, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Critchley, I.A.; Douglas, L.J. Role of glycosides as epithelial cell receptors for Candida albicans. J. Gen. Microbiol. 1987, 133, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Tosh, F.D.; Douglas, L.J. Characterization of a fucoside-binding adhesin of Candida albicans. Infect. Immun. 1992, 60, 4734–4739. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Lucho, V.; Ginsburg, V.; Krivan, H.C. Cryptococcus neoformans, Candida albicans, and other fungi bind specifically to the glycosphingolipid lactosylceramide (Galβl-4Glcβ3-lCer), a possible adhesion receptor for yeasts. Infect. Immun. 1990, 58, 2085–2090. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Lee, K.K.; Hodges, R.S.; Paranchych, W.; Irvin, R.T. Adherence of Pseudomonas aeruginosa and Candida albicans to Glycosphingolipid (Asialo-GM1) Receptors is Achieved by a Conserved Receptor-Binding Domain Present on Their Adhesins. Infect. Immun. 1994, 62, 5213–5219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, H.; Kavanagh, K.; Velasco-Torrijos, T. Targeting adhesion in fungal pathogen Candida albicans. Future Med. Chem. 2021, 13, 313–334. [Google Scholar] [CrossRef]
- Martin, H.; Goyard, D.; Margalit, A.; Doherty, K.; Renaudet, O.; Kavanagh, K.; Velasco-Torrijos, T. Multivalent Presentations of Glycomimetic Inhibitor of the Adhesion of Fungal Pathogen Candida albicans to Human Buccal Epithelial Cells. Bioconjug. Chem. 2021. ahead of print. [Google Scholar] [CrossRef]
- Roy, O.; Faure, S.; Thery, V.; Didierjean, C.; Taillefumier, C. Cyclic β-Peptoids. Org. Lett. 2008, 10, 921–924. [Google Scholar] [CrossRef]
- Cecioni, S.; Faure, S.; Darbost, U.; Bonnamour, I.; Parrot-Lopez, H.; Roy, O.; Taillefumier, C.; Wimmerová, M.; Praly, J.P.; Imberty, A.; et al. Selectivity among Two Lectins: Probing the Effect of Topology, Multivalency and Flexibility of “Clicked” Multivalent Glycoclusters. Chem. Eur. J. 2011, 17, 2146–2159. [Google Scholar] [CrossRef]
- Szekely, T.; Roy, O.; Dériaud, E.; Job, A.; Lo-Man, R.; Leclerc, C.; Taillefumier, C. Design, Synthesis, and Immunological Evaluation of a Multicomponent Construct Based on a Glycotripeptoid Core Comprising B and T Cell Epitopes and a Toll-like Receptor 7 Agonist That Elicits Potent Immune Responses. J. Med. Chem. 2018, 61, 9568–9582. [Google Scholar] [CrossRef] [PubMed]
- Tropper, F.D.; Andersson, F.O.; Braun, S.; Roy, R. Phase Transfer Catalysis as a General and Stereoselective Entry into Glycosyl Azides from Glycosyl Halides. Synthesis 1992, 1992, 618–620. [Google Scholar] [CrossRef]
- Lu, G.; Lam, S.; Burgess, K. An iterative route to “decorated” ethylene glycol-based linkers. Chem. Comm. 2006, 15, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Ng, D.Y.W.; Fahrer, J.; Wu, Y.; Eisele, K.; Kuan, S.L.; Barth, H.; Weil, T. Efficient Delivery of p53 and Cytochrome C by Supramolecular Assembly of a Dendritic Multi-Domain Delivery System. Adv. Healthc. Mater. 2013, 2, 1620–1629. [Google Scholar] [CrossRef] [PubMed]
- Norberg, O.; Deng, L.; Yan, M.; Ramström, O. Photo-Click Immobilization of Carbohydrates on Polymeric Surfaces—A Quick Method to Functionalize Surfaces for Biomolecular Recognition Studies. Bioconjug. Chem. 2009, 20, 2364–2370. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, H.; Masterson, H.; Kavanagh, K.; Velasco-Torrijos, T. The Synthesis and Evaluation of Multivalent Glycopeptoids as Inhibitors of the Adhesion of Candida albicans. Pathogens 2021, 10, 572. https://doi.org/10.3390/pathogens10050572
Martin H, Masterson H, Kavanagh K, Velasco-Torrijos T. The Synthesis and Evaluation of Multivalent Glycopeptoids as Inhibitors of the Adhesion of Candida albicans. Pathogens. 2021; 10(5):572. https://doi.org/10.3390/pathogens10050572
Chicago/Turabian StyleMartin, Harlei, Hannah Masterson, Kevin Kavanagh, and Trinidad Velasco-Torrijos. 2021. "The Synthesis and Evaluation of Multivalent Glycopeptoids as Inhibitors of the Adhesion of Candida albicans" Pathogens 10, no. 5: 572. https://doi.org/10.3390/pathogens10050572
APA StyleMartin, H., Masterson, H., Kavanagh, K., & Velasco-Torrijos, T. (2021). The Synthesis and Evaluation of Multivalent Glycopeptoids as Inhibitors of the Adhesion of Candida albicans. Pathogens, 10(5), 572. https://doi.org/10.3390/pathogens10050572