Sphingolipid Inhibitors as an Alternative to Treat Candidiasis Caused by Fluconazole-Resistant Strains
Abstract
:1. Introduction
2. Results
2.1. Antifungal Effect against Candida Strains
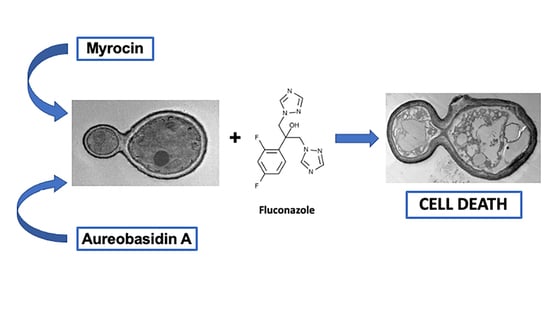
2.2. Interaction between Aureobasidin A, Myriocin, and Fluconazole
2.3. Cytotoxicity of Aureobasidin A and Myriocin
2.4. Effect of Aureobasidin A and Myriocin on the Lifespan of Wild Type Caenorhabditis elegans
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cell Lineages and Reagents
5.2. Susceptibility Tests with Aureobasidin A and Myriocin and Interaction with Fluconazole
5.3. Cytotoxicity Assay
5.4. Caenorhabditis elegans Lifespan Assay
5.5. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poulain, D. Candida albicans, plasticity and pathogenesis. Crit. Rev. Microbiol. 2015, 41, 208–217. [Google Scholar] [CrossRef]
- Dadar, M.; Tiwari, R.; Karthik, K.; Chakraborty, S.; Shahali, Y.; Dhama, K. Candida albicans-Biology, molecular characterization, pathogenicity, and advances in diagnosis and control-An update. Microb. Pathog. 2018, 117, 128–138. [Google Scholar] [CrossRef]
- Singh, A.; Rella, A.; Schwacke, J.; Vacchi-Suzzi, C.; Luberto, C.; Del Poeta, M. Transmembrane transporter expression regulated by the glucosylceramide pathway in Cryptococcus neoformans. BMC Res. Notes 2015, 8, 681. [Google Scholar] [CrossRef] [Green Version]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Kathiravan, M.K.; Salake, A.B.; Chothe, A.S.; Dudhe, P.B.; Watode, R.P.; Mukta, M.S.; Gadhwe, S. The biology and chemistry of antifungal agents: A review. Bioorg. Med. Chem. 2012, 20, 5678–5698. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.; Sherry, L.; Nile, C.J.; Sherriff, A.; Johnson, E.M.; Hanson, M.F.; Williams, C.; Munro, C.A.; Jones, B.J.; Ramage, G. Biofilm formation is a risk factor for mortality in patients with Candida albicans bloodstream infection-Scotland, 2012-2013. Clin. Microbiol. Infect. 2016, 22, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Patterson, T.F. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J. Infect. Dis. 2017, 216, S445–s451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roemer, T.; Krysan, D.J. Antifungal drug development: Challenges, unmet clinical needs, and new approaches. Cold Spring Harb. Perspect. Med. 2014, 4. [Google Scholar] [CrossRef]
- Dickson, R.C.; Lester, R.L. Sphingolipid functions in Saccharomyces cerevisiae. Biochim. Biophys. Acta 2002, 1583, 13–25. [Google Scholar] [CrossRef]
- Heung, L.J.; Luberto, C.; Del Poeta, M. Role of sphingolipids in microbial pathogenesis. Infect. Immun. 2006, 74, 28–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rollin-Pinheiro, R.; Singh, A.; Barreto-Bergter, E.; Del Poeta, M. Sphingolipids as targets for treatment of fungal infections. Future Med. Chem. 2016, 8, 1469–1484. [Google Scholar] [CrossRef] [Green Version]
- Rittershaus, P.C.; Kechichian, T.B.; Allegood, J.C.; Merrill, A.H., Jr.; Hennig, M.; Luberto, C.; Del Poeta, M. Glucosylceramide synthase is an essential regulator of pathogenicity of Cryptococcus neoformans. J. Clin. Investig. 2006, 116, 1651–1659. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Wang, M.; Wang, W.; Ruan, R.; Ma, H.; Mao, C.; Li, H. Glucosylceramides are required for mycelial growth and full virulence in Penicillium digitatum. Biochem. Biophys. Res. Commun. 2014, 455, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, C.M.; de Castro, P.A.; Singh, A.; Fonseca, F.L.; Pereira, M.D.; Vila, T.V.; Atella, G.C.; Rozental, S.; Savoldi, M.; Del Poeta, M.; et al. Functional characterization of the Aspergillus nidulans glucosylceramide pathway reveals that LCB Delta8-desaturation and C9-methylation are relevant to filamentous growth, lipid raft localization and Psd1 defensin activity. Mol. Microbiol. 2016, 102, 488–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oura, T.; Kajiwara, S. Candida albicans sphingolipid C9-methyltransferase is involved in hyphal elongation. Microbiology 2010, 156, 1234–1243. [Google Scholar] [CrossRef] [Green Version]
- Oura, T.; Kajiwara, S. Disruption of the sphingolipid Delta8-desaturase gene causes a delay in morphological changes in Candida albicans. Microbiology 2008, 154, 3795–3803. [Google Scholar] [CrossRef] [Green Version]
- Aeed, P.A.; Young, C.L.; Nagiec, M.M.; Elhammer, A.P. Inhibition of inositol phosphorylceramide synthase by the cyclic peptide aureobasidin A. Antimicrob. Agents Chemother. 2009, 53, 496–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerantola, V.; Guillas, I.; Roubaty, C.; Vionnet, C.; Uldry, D.; Knudsen, J.; Conzelmann, A. Aureobasidin A arrests growth of yeast cells through both ceramide intoxication and deprivation of essential inositolphosphorylceramides. Mol. Microbiol. 2009, 71, 1523–1537. [Google Scholar] [CrossRef] [Green Version]
- Lattif, A.A.; Mukherjee, P.K.; Chandra, J.; Roth, M.R.; Welti, R.; Rouabhia, M.; Ghannoum, M.A. Lipidomics of Candida albicans biofilms reveals phase-dependent production of phospholipid molecular classes and role for lipid rafts in biofilm formation. Microbiology 2011, 157, 3232–3242. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.W.; Tay, S.T. The inhibitory effects of aureobasidin A on Candida planktonic and biofilm cells. Mycoses 2013, 56, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Perdoni, F.; Signorelli, P.; Cirasola, D.; Caretti, A.; Galimberti, V.; Biggiogera, M.; Gasco, P.; Musicanti, C.; Morace, G.; Borghi, E. Antifungal activity of Myriocin on clinically relevant Aspergillus fumigatus strains producing biofilm. BMC Microbiol. 2015, 15, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazzarini, C.; Haranahalli, K.; Rieger, R.; Ananthula, H.K.; Desai, P.B.; Ashbaugh, A.; Linke, M.J.; Cushion, M.T.; Ruzsicska, B.; Haley, J.; et al. Acylhydrazones as Antifungal Agents Targeting the Synthesis of Fungal Sphingolipids. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [Green Version]
- Rocha, D.A.S.; Sa, L.F.R.; Pinto, A.C.C.; Junqueira, M.L.; Silva, E.M.D.; Borges, R.M.; Ferreira-Pereira, A. Characterisation of an ABC transporter of a resistant Candida glabrata clinical isolate. Mem. Inst. Oswaldo Cruz 2018, 113, e170484. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.C.C.; Rocha, D.A.S.; Moraes, D.C.; Junqueira, M.L.; Ferreira-Pereira, A. Candida albicans Clinical Isolates from a Southwest Brazilian Tertiary Hospital Exhibit MFS-mediated Azole Resistance Profile. An. Acad. Bras. Cienc. 2019, 91, e20180654. [Google Scholar] [CrossRef] [Green Version]
- White, T.C.; Holleman, S.; Dy, F.; Mirels, L.F.; Stevens, D.A. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob. Agents Chemother. 2002, 46, 1704–1713. [Google Scholar] [CrossRef] [Green Version]
- Almirante, B.; Rodríguez, D.; Park, B.J.; Cuenca-Estrella, M.; Planes, A.M.; Almela, M.; Mensa, J.; Sanchez, F.; Ayats, J.; Gimenez, M.; et al. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: Results from population-based surveillance, barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 2005, 43, 1829–1835. [Google Scholar] [CrossRef] [Green Version]
- Lackner, M.; Tscherner, M.; Schaller, M.; Kuchler, K.; Mair, C.; Sartori, B.; Istel, F.; Arendrup, M.C.; Lass-Flörl, C. Positions and numbers of FKS mutations in Candida albicans selectively influence in vitro and in vivo susceptibilities to echinocandin treatment. Antimicrob. Agents Chemother. 2014, 58, 3626–3635. [Google Scholar] [CrossRef] [Green Version]
- Zhong, W.; Jeffries, M.W.; Georgopapadakou, N.H. Inhibition of inositol phosphorylceramide synthase by aureobasidin A in Candida and Aspergillus species. Antimicrob. Agents Chemother. 2000, 44, 651–653. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Singh, A.; Kumari, S.; Kumar, P.; Wasi, M.; Mondal, A.K.; Rudramurthy, S.M.; Chakrabarti, A.; Gaur, N.A.; Gow, N.A.R.; et al. Sphingolipidomics of drug resistant Candida auris clinical isolates reveal distinct sphingolipid species signatures. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158815. [Google Scholar] [CrossRef]
- de Melo, N.R.; Abdrahman, A.; Greig, C.; Mukherjee, K.; Thornton, C.; Ratcliffe, N.A.; Vilcinskas, A.; Butt, T.M. Myriocin significantly increases the mortality of a non-mammalian model host during Candida pathogenesis. PLoS ONE 2013, 8, e78905. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Pei, Z.; Hu, R.; Zhang, Z.; Lou, Z.; Sun, X. Study on the Inhibitory Activity and Possible Mechanism of Myriocin on Clinically Relevant Drug-Resistant Candida albicans and Its Biofilms. Biol. Pharm. Bull. 2021, 44, 305–315. [Google Scholar] [CrossRef]
- Rollin-Pinheiro, R.; Rochetti, V.P.; Xisto, M.; Liporagi-Lopes, L.C.; Bastos, B.; Rella, A.; Singh, A.; Rozental, S.; Del Poeta, M.; Barreto-Bergter, E. Sphingolipid biosynthetic pathway is crucial for growth, biofilm formation and membrane integrity of Scedosporium boydii. Future Med. Chem. 2019, 11, 2905–2917. [Google Scholar] [CrossRef]
- Heidler, S.A.; Radding, J.A. The AUR1 gene in Saccharomyces cerevisiae encodes dominant resistance to the antifungal agent aureobasidin A (LY295337). Antimicrob. Agents Chemother. 1995, 39, 2765–2769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wuts, P.G.; Simons, L.J.; Metzger, B.P.; Sterling, R.C.; Slightom, J.L.; Elhammer, A.P. Generation of Broad-Spectrum Antifungal Drug Candidates from the Natural Product Compound Aureobasidin A. ACS Med. Chem. Lett. 2015, 6, 645–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandeputte, P.; Ferrari, S.; Coste, A.T. Antifungal resistance and new strategies to control fungal infections. Int. J. Microbiol. 2012, 2012, 713687. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.E.; Dismukes, W.E.; Duma, R.J.; Medoff, G.; Sande, M.A.; Gallis, H.; Leonard, J.; Fields, B.T.; Bradshaw, M.; Haywood, H.; et al. A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptoccal meningitis. N. Engl. J. Med. 1979, 301, 126–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado, A.; Casas, J.; Llebaria, A.; Abad, J.L.; Fabrias, G. Inhibitors of sphingolipid metabolism enzymes. Biochim. Biophys. Acta 2006, 1758, 1957–1977. [Google Scholar] [CrossRef]
- Streit, E.; Naehrer, K.; Rodrigues, I.; Schatzmayr, G. Mycotoxin occurrence in feed and feed raw materials worldwide: Long-term analysis with special focus on Europe and Asia. J. Sci. Food Agric. 2013, 93, 2892–2899. [Google Scholar] [CrossRef]
- de Sá, L.F.R.; Toledo, F.T.; de Sousa, B.A.; Gonçalves, A.C.; Tessis, A.C.; Wendler, E.P.; Comasseto, J.V.; Dos Santos, A.A.; Ferreira-Pereira, A. Synthetic organotelluride compounds induce the reversal of Pdr5p mediated fluconazole resistance in Saccharomyces cerevisiae. BMC Microbiol. 2014, 14, 201. [Google Scholar] [CrossRef] [Green Version]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Borenfreund, E.; Puerner, J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985, 24, 119–124. [Google Scholar] [CrossRef]
- Breger, J.; Fuchs, B.B.; Aperis, G.; Moy, T.I.; Ausubel, F.M.; Mylonakis, E. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog. 2007, 3, e18. [Google Scholar] [CrossRef] [PubMed]
- Tampakakis, E.; Okoli, I.; Mylonakis, E.A.C. Elegans-based, whole animal, in vivo screen for the identification of antifungal compounds. Nat. Protoc. 2008, 3, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Fatima, Z.; Ahmad, K.; Hameed, S. Fungicidal action of geraniol against Candida albicans is potentiated by abrogated CaCdr1p drug efflux and fluconazole synergism. PLoS ONE 2018, 13, e0203079. [Google Scholar] [CrossRef] [PubMed]




| Strains | Resistance Pattern | Reference |
|---|---|---|
| C. albicans (ATCC 10231D-5) | No resistance described | American Type Culture Collection * |
| C. glabrata (ATCC 2001D-5) | No resistance described | American Type Culture Collection * |
| C. glabrata (109) | CDR1 gene overexpressed (ABC transporter) | [24] |
| C. albicans (1114) | MDR1 gene overexpressed (MFS transporter) | [25] |
| C. albicans (12-99) | ERG11, CDR1, CDR2 and MDR1 genes overexpressed (ABC and MFS transporters) | [26] |
| * MIC90 (µg/mL) | |||
|---|---|---|---|
| Aureobasidin A | Myriocin | Fluconazole | |
| C. albicans (ATCC 10231D-5) | 0.5 | 2.0 | <8 |
| C. glabrata (ATCC 2001D-5) | 0.5 | 1.0 | <8 |
| C. glabrata (109) | 0.25 | 0.5 | >256 |
| C. albicans (1114) | 0.25 | 1.0 | >256 |
| C. albicans (12-99) | 0.25 | 0.25 | >256 |
| Candida Strains | |||
|---|---|---|---|
| 109 | 1114 | 12-99 | |
| MIC90 alone (µg/mL) | |||
| Fluconazole | >256 | 128 | >256 |
| Aureobasidin A | 0.25 | 0.25 | 0.25 |
| Myriocin | 0.5 | 1.0 | 0.25 |
| MIC90 combined (µg/mL) | |||
| Aureo/Fluco | 0.0156/32 | 0.03125/16 | 0.015/128 |
| Myr/Fluco | 0.0625/64 | 0.25/32 | 0.0625/16 |
| FICI | |||
| Aureo/Fluco | 0.1874 (synergic) | 0.25 (synergic) | 0.56 (no effect) |
| Myr/Fluco | 0.375 (synergic) | 0.5 (synergic) | 0.31 (synergic) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rollin-Pinheiro, R.; Bayona-Pacheco, B.; Domingos, L.T.S.; da Rocha Curvelo, J.A.; de Castro, G.M.M.; Barreto-Bergter, E.; Ferreira-Pereira, A. Sphingolipid Inhibitors as an Alternative to Treat Candidiasis Caused by Fluconazole-Resistant Strains. Pathogens 2021, 10, 856. https://doi.org/10.3390/pathogens10070856
Rollin-Pinheiro R, Bayona-Pacheco B, Domingos LTS, da Rocha Curvelo JA, de Castro GMM, Barreto-Bergter E, Ferreira-Pereira A. Sphingolipid Inhibitors as an Alternative to Treat Candidiasis Caused by Fluconazole-Resistant Strains. Pathogens. 2021; 10(7):856. https://doi.org/10.3390/pathogens10070856
Chicago/Turabian StyleRollin-Pinheiro, Rodrigo, Brayan Bayona-Pacheco, Levy Tenorio Sousa Domingos, Jose Alexandre da Rocha Curvelo, Gabriellen Menezes Migliani de Castro, Eliana Barreto-Bergter, and Antonio Ferreira-Pereira. 2021. "Sphingolipid Inhibitors as an Alternative to Treat Candidiasis Caused by Fluconazole-Resistant Strains" Pathogens 10, no. 7: 856. https://doi.org/10.3390/pathogens10070856
APA StyleRollin-Pinheiro, R., Bayona-Pacheco, B., Domingos, L. T. S., da Rocha Curvelo, J. A., de Castro, G. M. M., Barreto-Bergter, E., & Ferreira-Pereira, A. (2021). Sphingolipid Inhibitors as an Alternative to Treat Candidiasis Caused by Fluconazole-Resistant Strains. Pathogens, 10(7), 856. https://doi.org/10.3390/pathogens10070856