Molecular Detection of Trypanosoma spp. in Questing and Feeding Ticks (Ixodidae) Collected from an Endemic Region of South-West Australia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites, Sampling and Tick Identification
2.2. DNA Extraction and HRM-qPCR Detection of Trypanosoma spp.
2.3. Confirmation of qPCR Positive Results by DNA Sequencing
2.4. Statistical Analysis
3. Results
3.1. Tick Collection and Identification
3.2. HRM-qPCR Detection of Trypanosoma spp. in Questing and Feeding Ticks
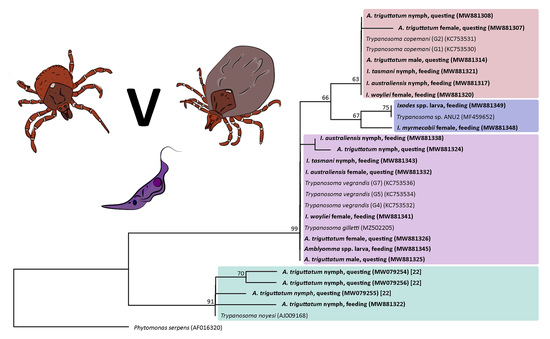
3.3. Sequence Based Identification of Trypanosoma spp. in Ticks
3.4. Statistical Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BLAST | Basic Local Alignment Search Tool |
| Bp | Base pairs |
| ML | Maximum-Likelihood |
| NCBI | National Center of Biotechnology Information |
| 18S rRNA | 18S ribosomal RNA |
References
- Hoare, C.A. The Trypanosomes of Mammals; Blackwell Scientific Publishing: Oxford, UK, 1972. [Google Scholar]
- Lazzari, C.R.; Pereira, M.H.; Lorenzo, M.G. Behavioural biology of Chagas disease vectors. Mem. Inst. Oswaldo Cruz 2013, 108 (Suppl. S1), 34–47. [Google Scholar] [CrossRef] [Green Version]
- Svobodová, M.; Dolnik, O.V.; Čepička, I.; Rádrová, J. Biting midges (Ceratopogonidae) as vectors of avian trypanosomes. Parasites Vectors 2017, 10, 224. [Google Scholar] [CrossRef]
- Geiger, A.; Malele, I.; Abd-Alla, A.M.; Njiokou, F. Blood feeding tsetse flies as hosts and vectors of mammals-pre-adapted African Trypanosoma: Current and expected research directions. BMC Microbiol. 2018, 18, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulandane, F.C.; Snyman, L.P.; Brito, D.R.A.; Bouyer, J.; Fafetine, J.; van den Abbeele, J.; Oosthuizen, M.; Delespaux, V.; Neves, L. Evaluation of the relative roles of the Tabanidae and Glossinidae in the transmission of trypanosomosis in drug resistance hotspots in Mozambique. Parasites Vectors 2020, 13, 219. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.K.; Godfrey, S.S.; Thompson, R.C.A. Trypanosomes of Australian mammals: A review. Int. J. Parasitol. Parasites Wildl. 2014, 3, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Cooper, C.; Clode, P.L.; Peacock, C.; Thompson, R.C.A. Host-parasite relationships and life histories of trypanosomes in Australia. Adv. Parasitol. 2016, 97, 47–109. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.; Reiss, A.; Jackson, B.; Warren, K.; Paparini, A.; Gillespie, G.; Stokeld, D.; Irwin, P.; Ryan, U. Prevalence, genetic diversity and potential clinical impact of blood-borne and enteric protozoan parasites in native mammals from northern Australia. Vet. Parasitol. 2017, 238, 94–105. [Google Scholar] [CrossRef]
- Northover, A.S.; Godfrey, S.S.; Keatley, S.; Lymbery, A.J.; Wayne, A.F.; Cooper, C.; Pallant, L.; Morris, K.; Thompson, R.C.A. Increased Trypanosoma spp. richness and prevalence of haemoparasite co-infection following translocation. Parasites Vectors 2019, 12, 126. [Google Scholar] [CrossRef]
- Egan, S.L.; Ruiz-Aravena, M.; Austen, J.M.; Barton, X.; Comte, S.; Hamilton, D.G.; Hamede, R.K.; Ryan, U.M.; Irwin, P.J.; Jones, M.E.; et al. Blood parasites in endangered wildlife—Trypanosomes discovered during a survey of haemoprotozoa from the Tasmanian devil. Pathogens 2020, 9, 873. [Google Scholar] [CrossRef]
- Ortiz-Baez, A.S.; Cousins, K.; Eden, J.-S.; Chang, W.-S.; Harvey, E.; Pettersson, J.H.-O.; Carver, S.; Polkinghorne, A.; Šlapeta, J.; Rose, K.; et al. Meta-transcriptomic identification of Trypanosoma spp. in native wildlife species from Australia. Parasites Vectors 2020, 13, 447. [Google Scholar] [CrossRef]
- Botero, A.; Thompson, C.K.; Peacock, C.S.; Clode, P.L.; Nicholls, P.K.; Wayne, A.F.; Lymbery, A.J.; Thompson, R.C.A. Trypanosomes genetic diversity, polyparasitism and the population decline of the critically endangered Australian marsupial, the brush tailed bettong or woylie (Bettongia penicillata). Int. J. Parasitol. Parasites Wildl. 2013, 2, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Wayne, A.F.; Maxwell, M.A.; Ward, C.G.; Vellios, C.V.; Wilson, I.J.; Wayne, J.C.; Williams, M.M.R. Sudden and rapid decline of the abundant marsupial Bettongia penicillata in Australia. Oryx 2015, 49, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.; Clark, P.; Averis, S.; Lymbery, A.J.; Wayne, A.F.; Morris, K.D.; Thompson, R.C.A. Trypanosomes in a declining species of threatened Australian marsupial, the brush-tailed bettong Bettongia penicillata (Marsupialia: Potoroidae). Parasitology 2008, 135, 1329–1335. [Google Scholar] [CrossRef] [Green Version]
- Cooper, C.; Keatley, S.; Northover, A.; Gofton, A.W.; Brigg, F.; Lymbery, A.J.; Pallant, L.; Clode, P.L.; Thompson, R.C.A. Next generation sequencing reveals widespread trypanosome diversity and polyparasitism in marsupials from Western Australia. Int. J. Parasitol. Parasites Wildl. 2018, 7, 58–67. [Google Scholar] [CrossRef]
- Apanaskevich, D.A.; Oliver, J.H., Jr. Life cycles and natural history of ticks. In Biology of Ticks, 2nd ed.; Sonenshine, D.E., Roe, R.M., Eds.; Oxford University Press: New York, NY, USA, 2013; Volume 1, pp. 59–73. [Google Scholar]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef]
- Krige, A.S.; Thompson, R.C.A.; Clode, P.L. ‘Hang on a Tick’—Are ticks really the vectors for Australian trypanosomes? Trends Parasitol. 2019, 35, 596–606. [Google Scholar] [CrossRef]
- Thompson, C.K.; Thompson, R.C.A. Trypanosomes of Australian mammals: Knowledge gaps regarding transmission and biosecurity. Trends Parasitol. 2015, 31, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Botero, A.; Cooper, C.; Thompson, C.K.; Clode, P.L.; Rose, K.; Thompson, R.C.A. Morphological and phylogenetic description of Trypanosoma noyesi sp. nov.: An Australian wildlife trypanosome within the T. cruzi clade. Protist 2016, 167, 425–439. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.C.A. Exotic parasite threats to Australia’s biosecurity—Trade, health, and conservation. Trop. Med. Infect. Dis. 2018, 3, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krige, A.S.; Thompson, R.C.A.; Seidlitz, A.; Keatley, S.; Botero, A.; Clode, P.L. ‘Hook, line, and sinker’: Fluorescence in situ hybridisation (FISH) uncovers Trypanosoma noyesi in Australian questing ticks. Ticks Tick Borne. Dis. 2021, 12, 101596. [Google Scholar] [CrossRef] [PubMed]
- Roberts, F.H.S. Australian Ticks; CSIRO Publishing: Melbourne, Australia, 1970. [Google Scholar]
- Barker, S.C.; Walker, A.R.; Campelo, D. A list of the 70 species of Australian ticks; diagnostic guides to and species accounts of Ixodes holocyclus (paralysis tick), Ixodes cornuatus (southern paralysis tick) and Rhipicephalus australis (Australian cattle tick); and consideration of the place of Australia in the evolution of ticks with comments on four controversial ideas. Int. J. Parasitol. 2014, 44, 941–953. [Google Scholar] [CrossRef]
- Keatley, S.; Botero, A.; Fosu-Nyarko, J.; Pallant, L.; Northover, A.; Thompson, R.C.A. Species-level identification of trypanosomes infecting Australian wildlife by High-Resolution Melting—Real Time Quantitative Polymerase Chain Reaction (HRM-qPCR). Int. J. Parasitol. Parasites Wildl. 2020, 13, 261–268. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Ash, A.; Elliot, A.; Godfrey, S.; Burmej, H.; Abdad, M.Y.; Northover, A.; Wayne, A.; Morris, K.; Clode, P.; Lymbery, A.; et al. Morphological and molecular description of Ixodes woyliei n. sp. (Ixodidae) with consideration for co-extinction with its critically endangered marsupial host. Parasites Vectors 2017, 10, 70. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, P.B.; Stevens, J.R.; Gidley, J.; Holz, P.; Gibson, W.C. A new lineage of trypanosomes from Australian vertebrates and terrestrial bloodsucking leeches (Haemadipsidae). Int. J. Parasitol. 2005, 35, 431–443. [Google Scholar] [CrossRef]
- Ellis, J.; Barratt, J.; Kaufer, A.; Pearn, L.; Armstrong, B.; Johnson, M.; Park, Y.; Downey, L.; Cao, M.; Neill, L.; et al. A new subspecies of Trypanosoma cyclops found in the Australian terrestrial leech Chtonobdella bilineata. Parasitology 2021, 148, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Paparini, A.; Irwin, P.J.; Warren, K.; McInnes, L.M.; de Tores, P.; Ryan, U.M. Identification of novel trypanosome genotypes in native Australian marsupials. Vet. Parasitol. 2011, 183, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Hall, J.; Rose, K.; Austen, J.; Egan, S.; Bilney, R.; Kambouris, P.; MacGregor, C.; Dexter, N. Baseline health parameters for a newly established population of long-nosed potoroo (Potorous tridactylus) at Booderee National Park, Australia. J. Wildl. Dis. 2021. [Google Scholar] [CrossRef]

| Tick Species | Life Stage | Total | Host Species | |||
|---|---|---|---|---|---|---|
| Larva | Nymph | Male | Female | |||
| Questing | ||||||
| Amblyomma triguttatum | 0 | 78 | 10 | 9 | 97 | n/a |
| Ixodes australiensis | 0 | 4 | 11 | 4 | 19 | n/a |
| Ixodes myrmecobii | 0 | 0 | 0 | 7 | 7 | n/a |
| Ixodes spp. | 25 | 0 | 0 | 0 | 25 | n/a |
| n/qPCR+/% | ||||||
| Total | 25/0/0.0 * | 82/30/36.6 | 21/8/38.1 | 20/8/40.0 | 148/46/31.1 | |
| DNA extracts | 125/46/36.8 | |||||
| Feeding | ||||||
| Amblyomma triguttatum | 0 | 1 | 0 | 0 | 1 | Woylie |
| Amblyomma spp. | 20 | 0 | 0 | 0 | 20 | Tammar wallaby, Woylie |
| Ixodes australiensis | 0 | 7 | 1 | 18 | 26 | Brush-tailed possum, Woylie |
| Ixodes fecialis | 0 | 0 | 0 | 1 | 1 | Quenda |
| Ixodes myrmecobii | 0 | 2 | 0 | 9 | 11 | Brush-tailed possum, Woylie |
| Ixodes tasmani | 0 | 9 | 0 | 1 | 10 | Brush-tailed possum |
| Ixodes woyliei | 0 | 2 | 0 | 11 | 13 | Woylie |
| Ixodes spp. | 1 | 0 | 0 | 0 | 1 | Woylie |
| n/qPCR+/% | ||||||
| Total | 21/8/38.0 * | 21/10/47.6 | 1/0/0.0 | 40/7/17.5 | 83/25/30.1 | |
| DNA extracts | 76/25/32.9 | |||||
| Questing Ticks | Feeding Ticks | |
|---|---|---|
| Trypanosoma copemani n (%) | 14 (11.2) | 6 (7.9) |
| Trypanosoma noyesi n (%) | 6 (4.8) [22] | 1 (1.3) |
| Trypanosoma vegrandis/T. gilletti n (%) | 13 (10.4) | 11 (14.5) |
| Trypanosoma sp. ANU2 n (%) | 2 (1.6) | 3 (3.9) |
| Total n (%) | 35/125 (28) | 21/76 (27.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krige, A.-S.; Thompson, R.C.A.; Seidlitz, A.; Keatley, S.; Wayne, J.; Clode, P.L. Molecular Detection of Trypanosoma spp. in Questing and Feeding Ticks (Ixodidae) Collected from an Endemic Region of South-West Australia. Pathogens 2021, 10, 1037. https://doi.org/10.3390/pathogens10081037
Krige A-S, Thompson RCA, Seidlitz A, Keatley S, Wayne J, Clode PL. Molecular Detection of Trypanosoma spp. in Questing and Feeding Ticks (Ixodidae) Collected from an Endemic Region of South-West Australia. Pathogens. 2021; 10(8):1037. https://doi.org/10.3390/pathogens10081037
Chicago/Turabian StyleKrige, Anna-Sheree, R. C. Andrew Thompson, Anke Seidlitz, Sarah Keatley, Julia Wayne, and Peta L. Clode. 2021. "Molecular Detection of Trypanosoma spp. in Questing and Feeding Ticks (Ixodidae) Collected from an Endemic Region of South-West Australia" Pathogens 10, no. 8: 1037. https://doi.org/10.3390/pathogens10081037
APA StyleKrige, A. -S., Thompson, R. C. A., Seidlitz, A., Keatley, S., Wayne, J., & Clode, P. L. (2021). Molecular Detection of Trypanosoma spp. in Questing and Feeding Ticks (Ixodidae) Collected from an Endemic Region of South-West Australia. Pathogens, 10(8), 1037. https://doi.org/10.3390/pathogens10081037