Antibacterial Agents Adsorbed on Active Carbon: A New Approach for S. aureus and E. coli Pathogen Elimination
Abstract
:1. Introduction
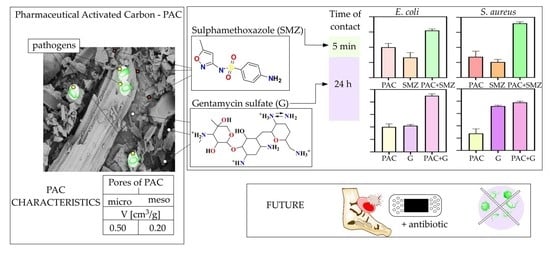
2. Results
2.1. PAC Characterization
2.2. Elemental Content of PAC
2.3. Adsorption of Antibacterial Agents on PAC
2.4. Adsorption Isotherms of Antibiotics on PAC
2.5. Bacterial Growth Reduction
2.6. Release of Antibacterial Agents from PAC
3. Discussion
4. Materials and Methods
4.1. Characteristics of PAC
4.2. Elemental Content of PAC
4.3. Adsorption of Antibacterial Agents on PAC
4.3.1. Adsorption of Gentamycin Sulfate (G) on PAC
4.3.2. Adsorption of Sulfamethoxazole (SMZ) on PAC
4.4. Adsorption Isotherms of Antibacterial Agents on PAC
4.4.1. Adsorption Isotherms of Gentamycin Sulfate (G) on PAC
4.4.2. Adsorption Isotherms for Sulfamethoxazole (SMZ) on PAC
4.5. Bacterial Growth Reduction by Adsorbed Gentamycin Sulfate (G) and Sulfamethoxazole (SMZ) on PAC
4.6. Release of Antibacterial Agents from PAC
4.6.1. Release of Gentamycin Sulfate from PAC under Aqueous Conditions
4.6.2. Release of Sulfamethoxazole from PAC under Aqueous Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sengupta, S.; Chattopadhyay, M.K.; Grossart, H.P. The multifaceted roles of antibiotics and antibiotic resistance in nature. Front. Microbiol. 2013, 4, 47. [Google Scholar] [CrossRef] [Green Version]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus 2017, 9, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Mobarki, N.; Almerabi, B.; Hattan, A. Antibiotic Resistance Crisis. Int. J. Med. Dev. Ctries. 2015, 40, 344–352. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.M.; Feil, E.J.; Smith, N.H. Population structure and evolutionary dynamics of pathogenic bacteria. BioEssays 2000, 12, 1115–1122. [Google Scholar] [CrossRef]
- Davies, S.C. Infections and the Rise of Antimicrobial Resistance. Annu. Rep. Chief Med. 2013, 381, 1606–1609. [Google Scholar]
- World Health Organization. Global Priority List of Antibiotic Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- World Health Organization. Model List of Essential Medicines; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Dalvi, R.R.; McGowan, C. Experimental induction of chronic aflatoxicosis in chickens by purified aflatoxin B1 and its reversal by activated charcoal, phenobarbital, and reduced glutathione. Poult. Sci. 1984, 63, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, R.R.; Ademoyero, A.A. Toxic Effects of Aflatoxin B 1 in Chickens Given Feed Contaminated with Aspergillus flavus and Reduction of the Toxicity by Activated Charcoal and Some Chemical Agents. Avian Dis. 1984, 28, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Sands, D.C.; McIntyre, J.L.; Walton, G.S. Use of activated charcoal for the removal of patulin from cider. Appl. Environ. Microbiol. 1976, 32, 388–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Souza, L.S.; Martins, A.C.; Silva, T.L.; Santos Júnior, O.O.; Visentainer, J.V.; Almeida, V.C. NaOH-activated carbon of high surface area produced from guava seeds as a high-efficiency adsorbent for amoxicillin removal: Kinetic, isotherm and thermodynamic studies. Chem. Eng. J. 2016, 288, 778–788. [Google Scholar] [CrossRef]
- Jung, M.W.; Ahn, K.H.; Lee, Y.; Kim, K.P.; Rhee, J.S.; Park, J.T.; Paeng, K.J. Adsorption characteristics of phenol and chlorophenols on granular activated carbons (GAC). Microchem. J. 2001, 70, 123–131. [Google Scholar] [CrossRef]
- Mohd Iqbaldin, M.N.; Khudzir, I.; Mohd Azlan, M.I.; Zaidi, A.G.; Surani, B.; Zubri, Z. Properties of coconut shell activated carbon. J. Trop. For. Sci. 2013, 25, 497–503. [Google Scholar]
- Bandosz, T.J.; Ania, C.O. Activated Carbon Surfaces in Environmental Remediation. Interface Sci. Technol. 2006, 7, 588. [Google Scholar]
- Chen, Y.D.; Chen, W.Q.; Huang, B.; Huang, M.J. Process optimization of K2C2O4-activated carbon from kenaf core using Box-Behnken design. Chem. Eng. Res. Des. 2013, 91, 1783–1789. [Google Scholar] [CrossRef]
- Lim, W.C.; Srinivasakannan, C.; Balasubramanian, N. Activation of palm shells by phosphoric acid impregnation for high yielding activated carbon. J. Anal. Appl. Pyrolysis. 2010, 88, 181–186. [Google Scholar] [CrossRef]
- Cao, Q.; Xie, K.C.; Lv, Y.K.; Bao, W.R. Process effects on activated carbon with large specific surface area from corn cob. Bioresour. Technol. 2006, 97, 110–115. [Google Scholar] [CrossRef]
- Khezami, L.; Ould-Dris, A.; Capart, R. Activated carbon from thermo-compressed wood and other lignocellulosic precursors. BioResources. 2007, 2, 193–209. [Google Scholar] [CrossRef]
- Ioannidou, O.; Zabaniotou, A. Agricultural residues as precursors for activated carbon production-A review. Renew. Sustain. Energy Rev. 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- Nowicki, P.; Pietrzak, R.; Wachowska, H. Sorption properties of active carbons obtained from walnut shells by chemical and physical activation. Catal. Today. 2010, 150, 107–114. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Bautista-Toledo, I.; Ferro-Garca, M.A.; Moreno-Castilla, C. Activated carbon surface modifications by adsorption of bacteria and their effect on aqueous lead adsorption. J. Chem. Technol. Biotechnol. 2001, 76, 120–1215. [Google Scholar] [CrossRef] [Green Version]
- Burchacka, E.; Łukaszewicz, M.; Kułażyński, M. Determination of mechanisms of action of active carbons as a feed additive. Bioorg. Chem. 2019, 93, 102804. [Google Scholar] [CrossRef]
- Mwafy, A.; Seyedzadeh, A.; Ahmed, W.K.; Ahmad, B.A.; Mathew, B.; Pandurangan, K.; Mourad, A.H.I.; Hilal-Alnaqbi, A. Adsorption of bilirubin toxin in liver by chitosan coated activated carbon prepared from date pits. In Proceedings of the Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics) Bioinformatics and Biomedical Engineering, 5th International Work-Conference, IWBBIO 2017, Granada, Spain, 26–28 April 2017. Proceedings, Part II, Editors: Rojas, Ignacio, Ortuño, Francisco (Eds.), LNBI 10208. [Google Scholar]
- Srivastava, V.C.; Mall, I.D.; Mishra, I.M. Adsorption of toxic metal ions onto activated carbon. Study of sorption behaviour through characterization and kinetics. Chem. Eng. Process. Process Intensif. 2008, 47, 1269–1280. [Google Scholar] [CrossRef]
- Yamamoto, S.; Ito, T.; Sato, M.; Goto, S.; Kazama, J.J.; Gejyo, F.; Narita, I. Adsorption of Protein-Bound Uremic Toxins Using Activated Carbon through Direct Hemoperfusion in vitro. Blood Purif. 2019, 48, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Sudheesh Kumar, P.T.; Lakshmanan, V.K.; Anilkumar, T.V.; Ramya, C.; Reshmi, P.; Unnikrishnan, A.G.; Nair, S.V.; Jayakumar, R. Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces. 2012, 4, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-H.; Hsu, H.-C.; Lin, C.-J. Treatment of Chronic Wounds With the Silver-Containing Activated Carbon Fiber Dressing: Three Cases. J. Med. Cases. 2014, 5, 587–591. [Google Scholar] [CrossRef]
- Beganovic, M.; Luther, M.K.; Rice, L.B.; Arias, C.A.; Rybak, M.J.; Laplante, K.L. A review of combination antimicrobial therapy for enterococcus faecalis bloodstream infections and infective endocarditis. Clin. Infect. Dis. 2018, 67, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Tacic, A.; Nikolic, V.; Nikolic, L.; Savic, I. Antimicrobial sulfonamide drugs. Adv. Technol. 2017, 6, 58–71. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, X.; Dong, W.; Zhang, L.; Kong, Q.; Wang, W. Efficient Adsorption of Sulfamethazine onto Modified Activated Carbon: A Plausible Adsorption Mechanism. Sci. Rep. 2017, 7, 1–12. [Google Scholar]
- Çalişkan, E.; Göktürk, S. Adsorption characteristics of sulfamethoxazole and metronidazole on activated carbon. Sep. Sci. Technol. 2010, 45, 244–255. [Google Scholar] [CrossRef]
- Baghdadi, M. UT (University of Tehran) isotherm as a novel and useful adsorption isotherm for investigation of adsorptive removal of pollutants. J. Environ. Chem. Eng. 2017, 5, 1906–1919. [Google Scholar] [CrossRef]
- Cardona, A.F.; Wilson, S.E. Skin and Soft-Tissue Infections: A Critical Review and the Role of Telavancin in Their Treatment. Clin. Infect. Dis. 2015, 15, 69–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petkovšek, Ž.; Eleršič, K.; Gubina, M.; Žgur-Bertok, D.; Erjavec, M.S. Virulence potential of Escherichia coli isolates from skin and soft tissue infections. J. Clin. Microbiol. 2009, 47, 1811–1817. [Google Scholar] [CrossRef] [PubMed]
- Tourmousoglou, C.E.; Yiannakopoulou, E.C.; Kalapothaki, V.; Bramis, J.; St. Papadopoulos, J. Surgical-Site Infection surveillance in general surgery: A critical issue. J. Chemother. 2008, 20, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Afifi, R.Y.; El-Hindawi, A.A. Acute necrotizing fasciitis in Egyptian patients: A case series. Int. J. Surg. 2008, 6, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Castilla, C.; López-Ramón, M.V.; Carrasco-Marín, F. Changes in surface chemistry of activated carbons by wet oxidation. Carbon N. Y. 2000, 38, 1995–2001. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Luque, R.; Campelo, J.M.; Colmenares, F.; Karpiński, Z.; Romero, A.A. Nanostructured photocatalysts and their applications in the photocatalytic transformation of lignocellulosic biomass: An overview. Materials 2009, 2, 2228–2258. [Google Scholar] [CrossRef]
- Lycklema, J. Adsorption from solution. Low molecular mass, uncharged molecules. In Fundamentals of Interface and Colloid Science, 1st ed.; Academic Press: Cambridge, MA, USA, 1995; pp. 1–59. [Google Scholar]
- Ngoddy, P.O.; Bakker-Arkema, F.W. A Generalized Theory of Sorption Phenomena in Biological Materials (Part I. The Isotherm Equation). Trans. ASAE 1970, 13, 612–617. [Google Scholar]
- Rizvi, S.S.H. Thermodynamic properties of foods in dehydration. In Engineering Properties of Foods, 3rd ed.; Taylor and Francis Group: Abingdon-on-Thames, UK, 2005. [Google Scholar]
- Dissemond, J.; Witthoff, M.; Brauns, T.C.; Haberer, D.; Goos, M. pH values in chronic wounds. Evaluation during modern wound therapy. Hautarzt 2003, 54, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Lorenc-Grabowska, E.; Diez, M.A.; Gryglewicz, G. Influence of pore size distribution on the adsorption of phenol on PET-based activated carbons. J. Colloid Interface Sci. 2016, 469, 205–212. [Google Scholar] [CrossRef]
- Bennett, G. The merck index: An encyclopedia of chemicals, drugs and biologicals. J. Hazard. Mater. 1992, 30, 361–381. [Google Scholar] [CrossRef]
- Pstrowska, K.; Kaczmarczyk, J.A.N.; Czapor-Irzabek, H.; Kułażyński, M. Characterization of activated beech wood char—Methane storage application. Stud. Univ. Babes-Bolyai Chem. 2018, 63, 21–39. [Google Scholar]
- Dubinin, M.M.; Radushkevich, L.V. The Equation of the Characteristic Curve of Activated Charcoal. Proc. Acad. Sci. Phys. Chem. Sect. 1947, 55, 331–337. [Google Scholar]
- Toda, Y.; Hatami, M.; Toyoda, S.; Yoshida, Y.; Honda, H. Micropore structure of coal. Fuel 1971, 50, 187–200. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Siemieniewska, T.; Albiniak, A.; Broniek, E.; Kaczmarczyk, J.; Jankowska, A.; McEnaney, B.; Chen, X.S.; Alain, E.; Furdin, G.; Bégin, D. Porosity development in steam activated chars from mixtures of coal tar pitch with graphite-FeCl3 intercalation compounds. Fuel 1998, 77, 509–517. [Google Scholar] [CrossRef]
- Pierce, C. Computation of pore sizes from physical adsorption data. J. Phys. Chem. 1953, 57, 149–152. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W.; Salzberg, H.W. Adsorption Surface Area and Porosity. J. Electrochem. Soc. 1967, 114, 279. [Google Scholar] [CrossRef]







| Pathogen List | Antibiotic- Resistant | Priority |
|---|---|---|
| Acinetobacter baumannii | carbapenem | CRITICAL |
| Pseudomonas aeruginosa | carbapenem | |
| Enterobacteriaceae * | carbapenem; 3rd-generation cephalosporin | |
| Enterococcus faecium | vancomycin | HIGH |
| Staphylococcus aureus | methicillin; vancomycin | |
| Helicobacter pylori | clarithromycin | |
| Campylobacter | fluoroquinolone | |
| Salmonella spp. | fluoroquinolone | |
| Neisseria gonorrhoeae | 3rd-generation cephalosporin, fluoroquinolone | |
| Streptococcus pneumoniae | penicillin | MEDIUM |
| Haemophilus influenzae | ampicillin | |
| Shigella spp. | fluoroquinolone |
| Parameter | Analysis Technique | Value |
|---|---|---|
| Moisture | drying method | 2.82 ± 0.22% analytical |
| Ash content | oven method | 0.78 ± 0.03% analytical 0.80 ± 0.03% dry |
| Volatile matter | oven method | 3.20 ± 0.03% analytical 3.32 ± 0.03% dry ash-free |
| pHPZC | Glass–electrode method | 7.010 |
| Carbon content | X-ray spectrophotometry | 93.6% weight |
| Specific surface area (BET) | benzene adsorption | 1267 m2/g |
| Micropore volume | thermogravimetric | 0.499 cm3/g |
| Mesopore volume | thermogravimetric | 0.205 cm3/g |
| Average width of mesopores | thermogravimetric | 4.69 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burchacka, E.; Pstrowska, K.; Beran, E.; Fałtynowicz, H.; Chojnacka, K.; Kułażyński, M. Antibacterial Agents Adsorbed on Active Carbon: A New Approach for S. aureus and E. coli Pathogen Elimination. Pathogens 2021, 10, 1066. https://doi.org/10.3390/pathogens10081066
Burchacka E, Pstrowska K, Beran E, Fałtynowicz H, Chojnacka K, Kułażyński M. Antibacterial Agents Adsorbed on Active Carbon: A New Approach for S. aureus and E. coli Pathogen Elimination. Pathogens. 2021; 10(8):1066. https://doi.org/10.3390/pathogens10081066
Chicago/Turabian StyleBurchacka, Ewa, Katarzyna Pstrowska, Elżbieta Beran, Hanna Fałtynowicz, Katarzyna Chojnacka, and Marek Kułażyński. 2021. "Antibacterial Agents Adsorbed on Active Carbon: A New Approach for S. aureus and E. coli Pathogen Elimination" Pathogens 10, no. 8: 1066. https://doi.org/10.3390/pathogens10081066
APA StyleBurchacka, E., Pstrowska, K., Beran, E., Fałtynowicz, H., Chojnacka, K., & Kułażyński, M. (2021). Antibacterial Agents Adsorbed on Active Carbon: A New Approach for S. aureus and E. coli Pathogen Elimination. Pathogens, 10(8), 1066. https://doi.org/10.3390/pathogens10081066