Investigation of Potential Gut Health Biomarkers in Broiler Chicks Challenged by Campylobacter jejuni and Submitted to a Continuous Water Disinfection Program
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Facilities, Biosecurity and Ethics
2.2. Experimental Design
2.3. Feed
2.4. Tested Product and Dosage Scheme
2.5. Challenge by Campylobacter jejuni
2.6. Serum Biomarkers
2.6.1. IL-10 and Cortisol Levels in the Serum
2.6.2. Fluorescein Isothiocyanate Dextran (FITC-d)
2.7. Faecal Biomarkers
2.7.1. Ovotransferrin
2.7.2. Whole Intestinal Transit Time (WITT) of Barium Sulfate
2.8. Biomarkers on Intestinal Tissue
2.8.1. Occludin (OCL), Toll-like Receptor-4 (TLR-4), and Mucin-2 (MUC2)
2.8.2. Gut Histomorphology
2.9. Biomarkers on Alimentary Content and Liver
2.10. Statistical Analysis
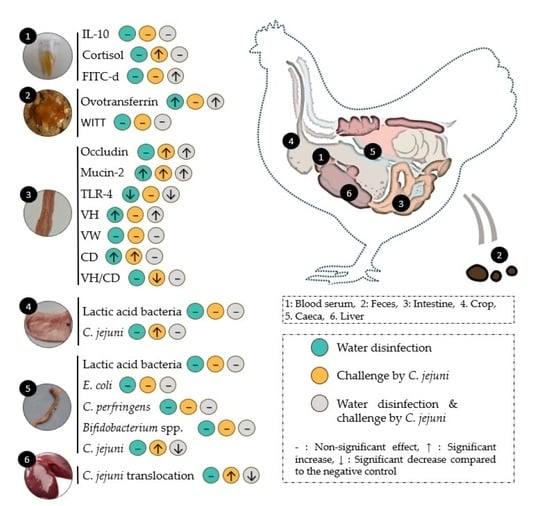
3. Results
3.1. Serum Biomarkers
3.2. Faecal Biomarkers
3.2.1. Ovotransferrin
3.2.2. Whole Intestinal Transit Time (WITT) of Barium Sulfate
3.3. Biomarkers on Intestinal Tissue
3.4. Biomarkers on Alimentary Content and Liver
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perera, T.R.W.; Skerrett-Byrne, D.A.; Gibb, Z.; Nixon, B.; Swegen, A. The Future of Biomarkers in Veterinary Medicine: Emerging Approaches and Associated Challenges. Animals 2022, 12, 2194. [Google Scholar] [CrossRef] [PubMed]
- Ducatelle, R.; Goossens, E.; De Meyer, F.; Eeckhaut, V.; Antonissen, G.; Haesebrouck, F.; Van Immerseel, F. Biomarkers for monitoring intestinal health in poultry: Present status and future perspectives. Vet. Res. 2018, 49, 43. [Google Scholar] [CrossRef] [PubMed]
- Rysman, K.; Eeckhaut, V.; Ducatelle, R.; Van Immerseel, F. The fecal biomarker ovotransferrin associates with broiler performance under field conditions. Poult. Sci. 2023, 102, 103011. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.F.A.; Merino-Guzman, R.; Latorre, J.D.; Mahaffey, B.D.; Yang, Y.; Teague, K.D.; Graham, L.E.; Wolfenden, A.D.; Hernandez-Velasco, X.; Bielke, L.R.; et al. Optimizing Fluorescein Isothiocyanate Dextran Measurement as a Biomarker in a 24-h Feed Restriction Model to Induce Gut Permeability in Broiler Chickens. Front. Vet. Sci. 2017, 4, 56. [Google Scholar] [CrossRef] [PubMed]
- Barekatain, R.; Howarth, G.S.; Willson, N.L.; Cadogan, D.; Wilkinson, S. Excreta biomarkers in response to different gut barrier dysfunction models and probiotic supplementation in broiler chickens. PLoS ONE 2020, 15, e0237505. [Google Scholar] [CrossRef] [PubMed]
- Igwaran, A.; Okoh, A.I. Human campylobacteriosis: A public health concern of global importance. Heliyon 2019, 5, e02814. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 12, e8442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Beyi, A.F.; Yin, Y. Zoonotic and antibiotic-resistant Campylobacter: A view through the One Health lens. One Health Adv. 2023, 1, 4. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Davies, R.; de Cesare, A.; Herman, L.; Hilbert, F.; et al. Update and review of control options for Campylobacter in broilers at primary production. EFSA J. 2020, 18, e06090. [Google Scholar] [CrossRef]
- Nauta, M.; Bolton, D.; Crotta, M.; Ellis-Iversen, J.; Alter, T.; Hempen, M.; Messens, W.; Chemaly, M. An Updated Assessment of the Effect of Control Options to Reduce Campylobacter Concentrations in Broiler Caeca on Human Health Risk in the European Union. Microb. Risk Anal. 2022, 21, 100197. [Google Scholar] [CrossRef]
- Hakeem, M.J.; Lu, X. Survival and Control of Campylobacter in Poultry Production Environment. Front. Cell. Infect. Microbiol. 2021, 10, 615049. [Google Scholar] [CrossRef] [PubMed]
- Alter, T. Prevention and Mitigation Strategies for Campylobacter with Focus on Poultry Production. In Campylobacter; Academic Press: Waltham, MA, USA, 2017; pp. 111–129. [Google Scholar] [CrossRef]
- Mantzios, T.; Tsiouris, V.; Kiskinis, K.; Economou, V.; Petridou, E.; Tsitsos, A.; Patsias, A.; Apostolou, I.; Papadopoulos, G.A.; Giannenas, I.; et al. In Vitro Investigation of the Antibacterial Activity of Nine Commercial Water Disinfectants, Acidifiers, and Glyceride Blends against the Most Important Poultry Zoonotic Bacteria. Pathogens 2023, 12, 381. [Google Scholar] [CrossRef] [PubMed]
- Mantzios, T.; Tsiouris, V.; Papadopoulos, G.A.; Economou, V.; Petridou, E.; Brellou, G.D.; Giannenas, I.; Biliaderis, C.G.; Kiskinis, K.; Fortomaris, P. Investigation of the Effect of Three Commercial Water Acidifiers on the Performance, Gut Health, and Campylobacter jejuni Colonization in Experimentally Challenged Broiler Chicks. Animals 2023, 13, 2037. [Google Scholar] [CrossRef] [PubMed]
- Guyard-Nicodeme, M.; Keita, A.; Quesne, S.; Amelot, M.; Poezevara, T.; Le Berre, B.; Sanchez, J.; Vesseur, P.; Martin, A.; Medel, P.; et al. Efficacy of feed additives against Campylobacter in live broilers during the entire rearing period. Poult. Sci. 2016, 95, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Drauch, V.; Ibesich, C.; Vogl, C.; Hess, M.; Hess, C. In-vitro testing of bacteriostatic and bactericidal efficacy of commercial disinfectants against Salmonella Infantis reveals substantial differences between products and bacterial strains. Int. J. Food Mic. 2020, 328, 108660. [Google Scholar] [CrossRef] [PubMed]
- Van Immerseel, F.; Russell, J.B.; Flythe, M.D.; Gantois, I.; Timbermont, L.; Pasmans, F.; Haesebrouck, F.; Ducatelle, R. The Use of Organic Acids to Combat Salmonella in Poultry: A Mechanistic Explanation of the Efficacy. Avian Pathol. 2006, 35, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Hess, C.; Hess, M. Re-thinking the chicken-Campylobacter jejuni interaction: A review. Avian Pathol. 2018, 47, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Goossens, E.; Debyser, G.; Callens, C.; Gussem, M.D.; Dedeurwaerder, A.; Devreese, B.; Haesebrouck, F. Elevated Faecal Ovotransferrin Concentrations Are Indicative for Intestinal Barrier Failure in Broiler Chickens. Vet. Res. 2018, 49, 1. [Google Scholar] [CrossRef]
- Aviagen. Ross-Broiler Management Handbook. 2018. Available online: https://en.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-BroilerHandbook2018-EN.pdf (accessed on 18 January 2024).
- Hermans, D.; Martel, A.; van Deun, K.; van Immerseel, F.; Heyndrickx, M.; Haesebrouck, F.; Pasmans, F. The cinnamon-oil ingredient trans-cinnamaldehyde fails to target Campylobacter jejuni strain KC 40 in the broiler chicken cecum despite marked in vitro activity. J. Food Prot. 2011, 74, 1729–1734. [Google Scholar] [CrossRef]
- Tsiouris, V.; Economou, E.; Lazou, T.; Georgopoulou, I.; Sossidou, E. The role of whey on the performance and campylobacteriosis in broiler chicks. Poult. Sci. 2019, 98, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Van Deun, K.; Pasmans, F.; Ducatelle, R.; Flahou, B.; Vissenberg, K.; Martel, A.; Van den Broeck, W.; Van Immerseel, F.; Haesebrouck, F. Colonization strategy of Campylobacter jejuni results in persistent infection of the chicken gut. Vet. Micr. 2008, 130, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, L.; Zheng, K.; Ma, Y.J.; He, R.X.; Arowolo, M.A.; Zhou, Y.J.; Xiao, D.F.; He, J.H. Effects of fenugreek seed extracts on growth performance and intestinal health of broilers. Poul. Sci. 2022, 101, 101939. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, W.H.; Lee, S.J.; Lillehoj, H.S. Detection of chicken interleukin-10 production in intestinal epithelial cells and necrotic enteritis induced by Clostridium perfringens using capture ELISA. Vet. Immunol. Immunopathol. 2018, 204, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Sand, J.M.; Arendt, M.K.; Repasy, A.; Deniz, G.; Cook, M.E. Oral antibody to interleukin-10 reduces growth rate depression due to Eimeria spp. infection in broiler chickens. Poul. Sci. 2016, 95, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Siswandi, R.; Yoshida, A.; Satoh, H.; Nonaka, N. X-ray evaluation of intestinal dysmotility induced by Eimeria pragensis infection in C57BL/6 mice. J. Vet. Med. Sci. 2019, 81, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Kolakshyapati, M.; Bailey, C.; Zimazile Sibanda, T.; Morgan, N.; Ruhnke, I. Determination of gastrointestinal passage rate using three different markers in laying hens. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1427–1436. [Google Scholar] [CrossRef]
- Chen, J.; Tellez, G.; Richards, J.D.; Escobar, J. Identification of Potential Biomarkers for Gut Barrier Failure in Broiler Chickens. Front. Vet. Sci. 2015, 2, 14. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Liu, D.; Guo, Y. Effects of Lactobacillus acidophilus on the growth performance and intestinal health of broilers challenged with Clostridium perfringens. J. Anim. Sci. Biotech. 2018, 9, 25. [Google Scholar] [CrossRef]
- Prakatur, I.; Miskulin, M.; Pavic, M.; Marjanovic, K.; Blazicevic, V.; Miskulin, I.; Domacinovic, M. Intestinal morphology in broiler chickens supplemented with propolis and bee pollen. Animals 2019, 9, 301. [Google Scholar] [CrossRef]
- Tsiouris, V.; Tassis, P.; Raj, J.; Mantzios, T.; Kiskinis, K.; Vasiljević, M.; Delić, N.; Petridou, E.; Brellou, G.D.; Polizopoulou, Z.; et al. Investigation of a Novel Multicomponent Mycotoxin Detoxifying Agent in Amelioration of Mycotoxicosis Induced by Aflatoxin-B1 and Ochratoxin A in Broiler Chicks. Toxins 2021, 13, 367. [Google Scholar] [CrossRef]
- ISO 10272-2:2017; Microbiology of the food chain Horizontal method for detection and enumeration of Campylobacter spp. ISO: Geneva, Switzerland, 2022.
- ISO 15214:1998; Microbiology of Food and Animal Feeding Stuffs Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria. ISO: Geneva, Switzerland, 2021.
- ISO 29981:2010Enumeration of Presumptive Bifidobacterial Colony Count Technique at 37 Degrees C, ISO: Geneva, Switzerland, 2015.
- ISO 16649-2:2001; Microbiology of Food and Animal Feeding Stuffs Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia Coli. ISO: Geneva, Switzerland, 2019.
- Tsiouris, V.; Georgopoulou, I.; Batzios, C.; Pappaioannou, N.; Ducatelle, R.; Fortomaris, P. High stocking density as a predisposing factor for necrotic enteritis in broiler chicks. Avian Pathol. 2015, 44, 59–66. [Google Scholar] [CrossRef]
- Aronson, J.K.; Ferner, R.E. Biomarkers—A General Review. Curr. Protoc. Pharmacol. 2017, 76, 9–23. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, T.; Rothwell, L.; Vervelde, L.; Kaiser, P.; Boulton, K.; Nolan, M.J.; Tomley, F.M.; Blake, D.P.; Hume, D.A. Analysis of the function of IL-10 in chickens using specific neutralising antibodies and a sensitive capture ELISA. Devel. Comp. Immunol. 2016, 63, 206–212. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Humphrey, S.; Chaloner, G.; Kemmett, K.; Davidson, N.; Williams, N.; Kipar, A.; Humphrey, T.; Wigley, P. Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare. mBio 2014, 5, e01364-14. [Google Scholar] [CrossRef]
- Reid, W.D.; Close, A.J.; Humphrey, S.; Chaloner, G.; Lacharme-Lora, L.; Rothwell, L.; Kaiser, P.; Williams, N.J.; Humphrey, T.J.; Wigley, P.; et al. Cytokine responses in birds challenged with the human food-borne pathogen Campylobacter jejuni implies a Th17 response. Roy. Soc. Open Sci. 2016, 3, 150541. [Google Scholar] [CrossRef]
- Mortada, M.; Cosby, D.E.; Akerele, G.; Ramadan, N.; Oxford, J.; Shanmugasundaram, R.; Ng, T.T.; Selvaraj, R.K. Characterizing the immune response of chickens to Campylobacter jejuni (Strain A74C). PLoS ONE 2021, 16, e0247080. [Google Scholar] [CrossRef]
- Chagneau, S.; Gaucher, M.-L.; Fravalo, P.; Thériault, W.P.; Thibodeau, A. Intestinal Colonization of Campylobacter jejuni and Its Hepatic Dissemination Are Associated with Local and Systemic Immune Responses in Broiler Chickens. Microorganisms 2023, 11, 1677. [Google Scholar] [CrossRef]
- Oluwagbenga, E.M.; Tetel, V.; Schober, J.; Fraley, G.S. Chronic Heat Stress Part 2: Increased Stress and Fear Responses in F1 Pekin Ducks Raised from Parents That Experienced Heat Stress. Animals 2023, 13, 1748. [Google Scholar] [CrossRef]
- Cockrem, J.F.; Candy, E.J.; Castille, S.A.; Satterlee, D.G. Plasma corticosterone responses to handling in Japanese quail selected for low or high plasma corticosterone responses to brief restraint. Br. Poult. Sci. 2010, 51, 453–459. [Google Scholar] [CrossRef]
- Flament, A.; Delleur, V.; Poulipoulis, A.; Marlier, D. Corticosterone, cortisol, triglycerides, aspartate aminotransferase and uric acid plasma concentrations during foie gras production in male mule ducks (Anas platyrhynchos × Cairina moschata). Br. Poult. Sci. 2012, 53, 408–413. [Google Scholar] [CrossRef]
- Gershon, M.D.; Margolis, K.G. The gut, its microbiome, and the brain: Connections and communications. J. Clin. Invest. 2021, 131, e143768. [Google Scholar] [CrossRef]
- Wickramasuriya, S. Gut Health of Poultry. Encyclopedia 2023. Available online: https://encyclopedia.pub/entry/18730 (accessed on 12 December 2023).
- Bull, S.A.; Thomas, A.; Humphrey, T.; Ellis-Iversen, J.; Cook, A.J.; Lovell, R.; Jorgensen, F. Flock health indicators and Campylobacter spp. in commercial housed broilers reared in Great Britain. Ap. Environ. Micr. 2008, 74, 5408–5413. [Google Scholar] [CrossRef]
- Colles, F.M.; Cain, R.J.; Nickson, T.; Smith, A.L.; Roberts, S.J.; Maiden, M.C.J.; Lunn, D.; Dawkins, M.S. Monitoring chicken flock behaviour provides early warning of infection by human pathogen Campylobacter. Proc. R. Soc. Lond. B 2016, 283, 20152323. [Google Scholar] [CrossRef]
- ACMSF (Advisory Committee for the Microbiological Safety of Foods). Second Report on Campylobacter. 2004. Available online: https://acmsf.food.gov.uk/sites/default/files/mnt/drupal_data/sources/files/multimedia/pdfs/acmsfcampylobacter.pdf (accessed on 12 December 2023).
- Alpigiani, I.; Abrahantes, J.C.; Michel, V.; Huneau-Salaün, A.; Chemaly, M.; Keeling, L.J.; Gervelmeyer, A.; Bacci, C.; Brindani, F.; Bonardi, S.; et al. Associations between animal welfare indicators and Campylobacter spp. in broiler chickens under commercial settings: A case study. Prev. Vet. Med. 2017, 147, 186–193. [Google Scholar] [CrossRef]
- Iyasere, O.S.; Beard, A.P.; Guy, J.H.; Bateson, M. Elevated levels of the stress hormone, corticosterone, cause ‘pessimistic’ judgment bias in broiler chickens. Sci. Rep. 2017, 7, 6860. [Google Scholar] [CrossRef]
- Truccollo, B.; Whyte, P.; Bolton, D.J. An Investigation of the Effect of Catecholamines and Glucocorticoids on the Growth and Pathogenicity of Campylobacter jejuni. Pathogens 2020, 9, 555. [Google Scholar] [CrossRef]
- Awad, W.A.; Hess, C.; Hess, M. Enteric Pathogens and Their Toxin-Induced Disruption of the Intestinal Barrier through Alteration of Tight Junctions in Chickens. Toxins 2017, 9, 60. [Google Scholar] [CrossRef]
- Cooper, C.A.; Tizard, M.L.; Stanborough, T.; Moore, S.C.; Chandry, P.S.; Jenkins, K.A.; Wise, T.G.; O’Neil, T.E.; Layton, D.S.; Morris, K.R.; et al. Overexpressing ovotransferrin and avian β-defensin-3 improves antimicrobial capacity of chickens and poultry products. Transgenic Res. 2019, 28, 51–76. [Google Scholar] [CrossRef]
- Marques, A.T.; Nordio, L.; Lecchi, C.; Grilli, G.; Giudice, C.; Ceciliani, F. Widespread extrahepatic expression of acute-phase proteins in healthy chicken (Gallus gallus) tissues. Vet. Immunol. Immunopathol. 2017, 190, 10–17. [Google Scholar] [CrossRef]
- Barekatain, R.; Chrystal, P.V.; Howarth, G.S.; McLaughlan, C.J.; Gilani, S.; Nattrass, G.S. Performance, intestinal permeability, and gene expression of selected tight junction proteins in broiler chickens fed reduced protein diets supplemented with arginine, glutamine, and glycine subjected to a leaky gut model. Poult. Sci. 2019, 98, 6761–6771. [Google Scholar] [CrossRef]
- Kuttappan, V.A.; Berghman, L.R.; Vicuña, E.A.; Latorre, J.D.; Menconi, A.; Wolchok, J.D.; Wolfenden, A.D.; Faulkner, O.B.; Tellez, G.I.; Hargis, B.M.; et al. Poultry enteric inflammation model with dextran sodium sulfate mediated chemical induction and feed restriction in broilers. Poult. Sci. 2015, 94, 1220–1226. [Google Scholar] [CrossRef]
- Gilani, S.; Howarth, G.S.; Kitessa, S.M.; Tran, C.D.; Forder, R.E.A.; Hughes, R.J. New biomarkers for increased intestinal permeability induced by dextran sodium sulphate and fasting in chickens. J. Anim. Phys. Anim. Nutr. 2017, 101, e237–e245. [Google Scholar] [CrossRef]
- Galarza-Seeber, R.; Latorre, J.D.; Bielke, L.R.; Kuttappan, V.A.; Wolfenden, A.D.; Hernandez-Velasco, X.; Merino-Guzman, R.; Vicente, J.L.; Donoghue, A.; Cross, D.; et al. Leaky Gut and Mycotoxins: Aflatoxin B1 Does Not Increase Gut Permeability in Broiler Chickens. Front. Vet. Sci. 2016, 3, 10. [Google Scholar] [CrossRef]
- Akerele, G.; Al Hakeem, W.G.; Lourenco, J.; Selvaraj, R.K. The Effect of Necrotic Enteritis Challenge on Production Performance, Cecal Microbiome, and Cecal Tonsil Transcriptome in Broilers. Pathogens 2022, 11, 839. [Google Scholar] [CrossRef]
- Graham, D.; Petrone-Garcia, V.M.; Hernandez-Velasco, X.; Coles, M.E.; Juarez-Estrada, M.A.; Latorre, J.D.; Chai, J.; Shouse, S.; Zhao, J.; Forga, A.J.; et al. Assessing the effects of a mixed Eimeria spp. challenge on performance, intestinal integrity, and the gut microbiome of broiler chickens. Front. Vet. Sci. 2023, 10, 1224647. [Google Scholar] [CrossRef]
- McKenzie, M.E.; Colnago, G.L.; Lee, S.R.; Long, P.L. Gut stasis in chickens infected with Eimeria. Poult. Sci. 1987, 66, 264–269. [Google Scholar] [CrossRef]
- Bloch, R.A.; Cronin, K.; Hoover, J.P.; Pechman, R.D.; Payton, M.E. Evaluation of gastrointestinal tract transit times using barium-impregnated polyethylene spheres and barium sulfate suspension in a domestic pigeon (Columba livia) model. J. Av. Med. Surg. 2010, 24, 1–8. [Google Scholar] [CrossRef]
- Mustafa, A.; Bai, S.; Zeng, Q.; Ding, X.; Wang, J.; Xuan, Y.; Su, Z.; Zhang, K. Effect of organic acids on growth performance, intestinal morphology, and immunity of broiler chickens with and without coccidial challenge. A.M.B. Express 2021, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Rzeznitzeck, J.; Breves, G.; Rychlik, I.; Hoerr, F.J.; von Altrock, A.; Rath, A.; Rautenschlein, S. The effect of Campylobacter jejuni and Campylobacter coli colonization on the gut morphology, functional integrity, and microbiota composition of female turkeys. Gut Pathog. 2022, 14, 33. [Google Scholar] [CrossRef]
- Kwon, O.; Han, T.S.; Son, M.Y. Intestinal morphogenesis in development, regeneration, and disease: The potential utility of intestinal organoids for studying compartmentalization of the crypt-villus structure. Front. Cell Devel. Biol. 2020, 8, 593969. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.N.; Le, N.H.; Pham, V.V.; Eva, P.; Alberto, F.; Le, H.T. Relationship between the ratio of villous height:crypt depth and gut bacteria counts as well production parameters in broiler chickens. J. Agricul. Devel. 2021, 20, 1–10. [Google Scholar] [CrossRef]
- Iwashita, J.; Yukita, S.; Hiroko, S.; Nagatomo, T.; Hiroshi, S.; Tatsuya, A. mRNA of MUC2 is stimulated by IL-4, IL-13 or TNF-α through a mitogen-activated protein kinase pathway in human colon cancer cells. Immun. Cell Biol. 2003, 81, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Horn, N.L.; Donkin, S.S.; Applegate, T.J.; Adeola, O. Intestinal mucin dynamics, response of broiler chicks and white pekin ducklings to dietary threonine. Poul. Sci. 2009, 88, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Sewid, A.H.; Arisha, A.H.; Abd El-Fattah, A.H.; Abdelaziz, A.M.; Al-Jabr, O.A.; Kishawy, A.T.Y. Influence of Glycyrrhiza glabra Extract on Growth, Gene Expression of Gut Integrity, and Campylobacter jejuni Colonization in Broiler Chickens. Front. Vet. Sci. 2020, 7, 612063. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; El-Daly, E.F.; El-Azeem, N.A.A.; Youssef, A.W.; Hassan, H. Growth performance and histological changes in ileum and immune related organs of broilers fed organic acids or antibiotic growth promoter. Int. J. Poult. Sci. 2014, 13, 602–610. [Google Scholar] [CrossRef]
- Paul, S.K.; Halder, G.; Mondal, M.K.; Samanta, G. Effect of organic acid salt on the performance and gut health of broiler chicken. Poult. Sci. 2007, 44, 389–395. [Google Scholar] [CrossRef]
- Onderci, M.; Sahin, N.; Sahin, K.; Cikim, G.; Aydin, A.; Ozercan, I. Efficacy of supplementation of alpha-amylase-producing bacterial culture on the performance, nutrient use and gut morphology of broiler chickens fed a corn-based diet. Poult. Sci. 2006, 85, 505–510. [Google Scholar] [CrossRef]
- Samanya, M.; Yamauchi, K. Histological alterations of intestinal villi in chickens fed dried Bacillus subtilis var. natto. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 133, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Izadi, H.; Arshami, J.; Golian, A.; Raji, M.R. Effects of chicory root powder on growth performance and histomorphometry of jejunum in broiler chicks. Vet. Res. Forum 2013, 4, 169–174. [Google Scholar] [PubMed]
- Van Nevel, C.J.; Decuypere, J.A.; Dierick, N.A.; Molly, K. Incorporation of galactomannans in the diet of newly weaned piglets, effect on bacteriological and some morphological characteristics of the small intestine. Arch. Anim. Nutr. 2005, 59, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.; Smyth, J.A.; McCracken, K.J. Digestive development of the early-weaned pig. 1. Effect of continuous nutrient supply on the development of the digestive tract and on changes in digestive enzyme activity during the first week post-weaning. Br. J. Nutr. 1991, 65, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.A.; Richardson, L.J.; Buhr, R.J.; Fedorka-Cray, P.J. Campylobacter species occurrence within internal organs and tissues of commercial caged Leghorn laying hens. Poult. Sci. 2009, 88, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Dublecz, F.; Hess, C.; Dublecz, K.; Khayal, B.; Aschenbach, J.R.; Hess, M. Campylobacter jejuni colonization promotes the translocation of Escherichia coli to extra-intestinal organs and disturbs the short-chain fatty acids profiles in the chicken gut. Poult. Sci. 2016, 95, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Ge, Z.; Fox, J.G.; Schauer, D.B. Disruption of tight junctions and induction of proinflammatory cytokine responses in colonic epithelial cells by Campylobacter jejuni. Infect. Immun. 2006, 74, 6581–6589. [Google Scholar] [CrossRef] [PubMed]
- Wine, E.; Chan, V.L.; Sherman, P.M. Campylobacter jejuni mediated disruption of polarized epithelial monolayers is cell-type specific, time dependent, and correlates with bacterial invasion. Pediatr. Res. 2008, 64, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Emami, N.K.; Calik, A.; White, M.B.; Young, M.; Dalloul, R.A. Necrotic Enteritis in Broiler Chickens: The Role of Tight Junctions and Mucosal Immune Responses in Alleviating the Effect of the Disease. Microorganisms 2019, 7, 231. [Google Scholar] [CrossRef]
- Kuo, W.T.; Shen, L.; Zuo, L.; Shashikanth, N.; Ong, M.L.D.M.; Wu, L.; Zha, J.; Edelblum, K.L.; Wang, Y.; Wang, Y.; et al. Inflammation-induced Occludin Downregulation Limits Epithelial Apoptosis by Suppressing Caspase-3 Expression. Gastroenterology 2019, 157, 1323–1337. [Google Scholar] [CrossRef]
- Márquez, M.; Fernández Gutiérrez Del Álamo, C.; GirónGonzález, J.A. Gut epithelial barrier dysfunction in human immunodeficiency virus-hepatitis C virus coinfected patients: Influence on innate and acquired immunity. World J. Gastroenterol. 2016, 22, 1433–1448. [Google Scholar] [CrossRef]
- Ferronato, G.; Prandini, A. Dietary Supplementation of Inorganic, Organic, and Fatty Acids in Pig: A Review. Animals 2020, 10, 1740. [Google Scholar] [CrossRef]
- Ebeid, T.; Al-Homidan, I.; Fathi, M.; Al-Jamaan, R.; Mostafa, M.; Abou-Emera, O.; El-Razik, M.A.; Alkhalaf, A. Impact of probiotics and/or organic acids supplementation on growth performance; microbiota; antioxidative status; and immune response of broilers. It. J. Anim. Sci. 2021, 20, 2263–2273. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Yan, F.; Yang, C.; Yang, X. Effects of encapsulated organic acids and essential oils on intestinal barrier, microbial count, and bacterial metabolites in broiler chickens. Poult. Sci. 2019, 98, 2858–2865. [Google Scholar] [CrossRef] [PubMed]
- Palamidi, I.; Mountzouris, K.C. Diet supplementation with an organic acids-based formulation affects gut microbiota and expression of gut barrier genes in broilers. Anim. Nutr. 2018, 4, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Qi, G.; Wang, J.; Zhang, H.; Qiu, K.; Han, Y.; Wu, Y.; Wu, S. Dietary organic acids ameliorate high stocking density stress-induced intestinal inflammation through the restoration of intestinal microbiota in broilers. J. Animal. Sci. Biotechnol. 2022, 13, 124. [Google Scholar] [CrossRef]
- Polycarpo, G.V.; Andretta, I.; Kipper, M.; Cruz-Polycarpo, V.C.; Dadalt, J.C.; Rodrigues, P.H.M.; Albuquerque, R. Meta-analytic study of organic acids as an alternative performance-enhancing feed additive to antibiotics for broiler chickens. Poult. Sci. 2017, 96, 3645–3653. [Google Scholar] [CrossRef]





| Genes | Forward Primer | Reserve Primer | Fragment Size (bp) |
|---|---|---|---|
| OCL | 5′-GAGCCCAGACTACCAAAGCAA-3′ | 5′-GCTTGATGTGGAAGAGCTTGTTG-3′ | 68 |
| TLR-4 | 5′-CAGCACCAACTTCTCAGTTCC-3′ | 5′-TCTGCAGCCACACATTCTTT-3′ | 63 |
| MUC2 | 5′-CCACACACCTGCCTACATGAA-3′ | 5′-GGATGGCAAGAGGACATATCAAA-3′ | 102 |
| GAPDH | 5′-CTGGCAAAGTCCAAGTGGTG-3′ | 5′-CCCTTGAAGTGTCCGTGTGT-3′ | 100 |
| Bacterium | Group A (Negative Control) | Group B (Cid 2000™) | Group C (C. jejuni) | Group D (Cid 2000™ and C. jejuni) | p Value |
|---|---|---|---|---|---|
| Crop | |||||
| C. jejuni | ND | ND | 6.43 ± 0.25 | 6.39 ± 0.10 | 0.880 |
| Lactic acid bacteria | 8.45 ± 0.09 | 8.57 ± 0.09 | 8.80 ± 0.11 | 8.46 ± 0.18 | 0.170 |
| Ceca | |||||
| C. jejuni | ND | ND | 7.84 ± 0.12 b | 7.10 ± 0.23 a | 0.010 |
| E. coli | 6.33 ± 0.16 | 6.35 ± 0.21 | 6.51 ± 0.23 | 6.00 ± 0.16 | 0.302 |
| C. perfringens | 1.38 ± 0.22 | 1.48 ± 0.18 | 1.44 ± 0.19 | 1.36 ± 0.15 | 0.966 |
| Lactic acid bacteria | 7.13 ± 0.21 | 7.10 ± 0.22 | 7.46 ± 0.22 | 7.40 ± 0.17 | 0.508 |
| Bifidobacterium spp. | 6.46 ± 0.14 | 6.52 ± 0.16 | 6.66 ± 0.08 | 6.62 ± 0.18 | 0.747 |
| C. jejuni | Group A (Negative Control) | Group B (Cid 2000™) | Group C (C. jejuni) | Group D (Cid 2000™ and C. jejuni) | p (Chi2) |
|---|---|---|---|---|---|
| Not detected | 100% | 100% | 18.8% | 62.5% | |
| Detected | 0% | 0% | 81.2% | 37.5% | |
| Total | 100% | 100% | 100% | 100% | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantzios, T.; Kiousi, D.E.; Brellou, G.D.; Papadopoulos, G.A.; Economou, V.; Vasilogianni, M.; Kanari, E.; Petridou, E.; Giannenas, I.; Tellez-Isaias, G.; et al. Investigation of Potential Gut Health Biomarkers in Broiler Chicks Challenged by Campylobacter jejuni and Submitted to a Continuous Water Disinfection Program. Pathogens 2024, 13, 356. https://doi.org/10.3390/pathogens13050356
Mantzios T, Kiousi DE, Brellou GD, Papadopoulos GA, Economou V, Vasilogianni M, Kanari E, Petridou E, Giannenas I, Tellez-Isaias G, et al. Investigation of Potential Gut Health Biomarkers in Broiler Chicks Challenged by Campylobacter jejuni and Submitted to a Continuous Water Disinfection Program. Pathogens. 2024; 13(5):356. https://doi.org/10.3390/pathogens13050356
Chicago/Turabian StyleMantzios, Tilemachos, Despoina E. Kiousi, Georgia D. Brellou, Georgios A. Papadopoulos, Vangelis Economou, Marili Vasilogianni, Elisavet Kanari, Evanthia Petridou, Ilias Giannenas, Guillermo Tellez-Isaias, and et al. 2024. "Investigation of Potential Gut Health Biomarkers in Broiler Chicks Challenged by Campylobacter jejuni and Submitted to a Continuous Water Disinfection Program" Pathogens 13, no. 5: 356. https://doi.org/10.3390/pathogens13050356
APA StyleMantzios, T., Kiousi, D. E., Brellou, G. D., Papadopoulos, G. A., Economou, V., Vasilogianni, M., Kanari, E., Petridou, E., Giannenas, I., Tellez-Isaias, G., Pappa, A., Galanis, A., & Tsiouris, V. (2024). Investigation of Potential Gut Health Biomarkers in Broiler Chicks Challenged by Campylobacter jejuni and Submitted to a Continuous Water Disinfection Program. Pathogens, 13(5), 356. https://doi.org/10.3390/pathogens13050356