2.1. Architecture of Salmonella enterica Biofilms
All strains of
S. enterica formed biofilm on polystyrene microtiter plates after 24 h of incubation at 37 °C. The untreated control biofilms presented values varying between serotypes in respect of biovolume in the observation field of 14.2 × 10
3 µm
2 (running from 8.2 × 10
3 ± 1.3 × 10
3 to 76.5 × 10
3 ± 34.1 × 10
3 µm
3;
Table 1), percentage of surface coverage (from 29.1 ± 2.8% to 96.9 ± 3.8%;
Table 2), maximum thickness (between 15.7 ± 2.1 and 53.3 ± 13.8 µm;
Table 3), and roughness (going from 0.442 ± 0.060 to 0.682 ± 0.048;
Table 4). The serotypes
S. Kentucky and
S. Hadar showed a trend to produce biofilms with the greatest biovolume, with values in the field observed (14.2 × 10
3 µm
2) of 76.5 × 10
3 ± 34.1 × 10
3 and 71.8 × 10
3 ± 24.8 × 10
3 µm
3, respectively. These were followed (
P > 0.05) by
S. Anatum (54.0 × 10
3 ± 11.7 × 10
3 µm
3) and
S. Typhimurium (47.5 × 10
3 ± 6.3 × 10
3 µm
3). Biofilms of these four serotypes also showed a trend to have the highest percentage of surface coverage (between 88.9 ± 6.2% and 96.9 ± 3.8%) and the greatest thickness (running from 34.7 ± 4.5 to 53.3 ± 13.8 µm).
S. Enteritidis and
S. Typhimurium monophasic variant 1,4,(5),12:i:- showed lower figures (
P < 0.01) for biovolume, percentage of surface covered, and maximum thickness (12.9 × 10
3 ± 9.3 × 10
3 µm
3, 32.4 ± 6.8%, and 18.3 ± 4.3 µm, respectively, as average) than the rest of the serotypes (44.4 × 10
3 ± 24.7 × 10
3 µm
3, 83.2 ± 12.1%, and 34.6 ± 12.2 µm, respectively).
S. Enteritidis and
S. 1,4,(5),12:i:-, unlike the others serotypes, formed only microcolonies of non-confluent cells after twenty-four hours of incubation at 37 °C (
Figure 1).
The fact that all strains of
S. enterica studied had the ability to produce biofilm on polystyrene surfaces is a cause for concern in the context of food safety and public health. This is because the plastic in question is material in very wide use on cattle farms, in slaughterhouses, in food-processing plants, and in establishments serving food. It is frequently used to manufacture a range of surfaces such as piping, cutting boards, and other equipment [
15,
16]. Moreover, a number of researchers have demonstrated that there is a positive correlation between the production of biofilm on polystyrene microtiter plates and the formation of biofilms on various materials used for surfaces in the food industry [
17,
18].
The formation of biofilms is one of the commonest strategies used by bacteria in tolerating various sorts of environmental stress [
19]. Biofilms increase microbial resistance to physical, biological and chemical agents (for example, antimicrobials), so that the capacity to form biofilms contributes greatly to the persistence of bacteria in food-processing installations [
18,
20]. Indeed, molecular techniques have been used to show that certain strains of
Salmonella can remain for several years in food-processing plants [
21]. Furthermore, biofilms that are formed in food-processing environments pose a major problem for the food industry, as these structures have been identified as the principal source of contamination of foodstuffs with pathogenic and spoilage microorganisms. This fact constitutes a challenge for public health and involves considerable financial losses [
22]. For instance, on these lines it has been shown that the percentage of poultry carcasses contaminated with
Salmonella grows significantly during processing, as a consequence of the presence of bacteria on surfaces with which these foods come into contact [
23].
The present study found striking differences between serotypes with regard to their capacity to form biofilm, as had also been observed previously [
11,
12,
18].
S. Kentucky and
S. Hadar formed strong biofilms (high values for biovolume, percentage of surface covered, and maximum thickness). The fact that
S. Kentucky is a powerful producer of biofilm is particularly noteworthy, as this serotype is characterized by normally having a high level of resistance to antibiotics of clinical importance, such as ciprofloxacin [
24]. The results of the research being reported here do not coincide completely with the findings from previous investigations, in which it was observed that
Salmonella Hadar (strain SH174) had only a slight ability to form biofilm on polystyrene after 24 h of incubation (15.3 × 10
3 ± 4.7 × 10
3 µm
3 in the observation field of 14.2 × 10
3 µm
2) [
25]. Similarly, it is not in agreement with the observations of other researchers indicating that
S. Kentucky and
S. Hadar are moderate producers of biofilm [
26]. These discrepancies among research may be due to variations between different strains of the same serotype with regard to their abilities to form biofilm [
11].
The strain of
S. Typhimurium tested was a strong producer of biofilm. The considerable capacity of strains of this serotype to form biofilm on polystyrene has also been highlighted in previous work [
27] in which, after 24 h of incubation on glass at 37 °C, biofilms with a biovolume of 129.4 × 10
3 ± 34.7 × 10
3 µm
3 were seen in the field of observation (14.2 × 10
3 µm
2) for another strain of
S. Typhimurium (strain S175). The great ability to form biofilm that was seen in
S. Typhimurium in the present study is a finding of considerable interest from the viewpoint of public health, as this serotype is among the most dangerous to humans. Sarwari et al. [
28] developed a mathematical model for predicting the capacity to cause human illness, and
Salmonella Typhimurium attained the highest score of the seven serotypes compared [
29]. On the other hand, some authors have noted that the strains of this serotype produce little biofilm on microtiter plates and do so slowly [
18].
Several researchers have demonstrated that strains of
Salmonella Agona have a substantial capacity to form biofilm [
11,
30]. However, the strain of
S. Agona trialed in the present work was among those producing the least biofilm. Finally, strains of the serotypes
S. Infantis,
S. Virchow, and
S. Enteritidis are, in general, poor at producing biofilm [
31], this fact providing backing for some of the findings of the study being reported here.
2.2. Effect of Differing Concentrations of SHY and BZK on Biofilms of Salmonella enterica
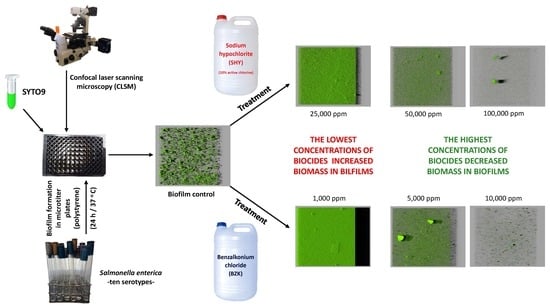
Biofilms of Salmonella formed on polystyrene after 24 h of incubation at 37 °C were exposed for 10 min to aqueous solutions of SHY (25,000, 50,000, or 100,000 ppm) or BZK (1000, 5000, or 10,000 ppm).
Use of SHY at 25,000 ppm (2500 ppm of active chlorine) did not reduce the biovolume or the surface coverage of the biofilms in any case. Indeed, for some strains (
S. Enteritidis and
S. 1,4,(5),12:i-) this treatment was even associated with an increase in biovolume and in the percentage of surface coverage of the biofilm (
Table 1 and
Table 2,
Figure 1). Even though additional research studies are needed to support these findings, our results suggest that the concentrations of SHY habitually used in disinfecting equipment and installations (800 to 2000 ppm of active chlorine) [
9,
32] could fail to be effective in eliminating biofilms of
Salmonella when the disinfectant is applied for a ten-minute period. On these lines, Holah [
33] indicated that exposure times in excess of ten minutes are advisable when disinfecting utensils or equipment, especially when they are hard for disinfectants to reach. Furthermore, it should be noted that there are circumstances in which disinfectants are applied in small doses, for instance as the consequence of an inaccurate calculation of concentrations, inappropriate storage of the chemicals, the difficulty of reaching certain locations, or the presence of excessive quantities of organic matter, which reduces the effectiveness of some biocides, such as chlorine compounds [
34]. When SHY was applied at 50,000 or 100,000 ppm (5000 or 10,000 ppm of active chlorine, respectively), reductions in biovolume and in the percentage of surface covered by the biofilm were observed relative to the control samples in the majority of cases (
Table 1 and
Table 2,
Figure 1).
One noteworthy feature of this work is the increase in biomass observed after exposure to BZK at 1000 ppm. This treatment caused a significant growth in the biovolume of films relative to unexposed strains in
S. Agona,
S. Enteritidis,
S. Thompson, and
S. 1,4,(5),12:i:- (
Table 5,
Figure 2). BZK at 1000 ppm brought about an increase (
P < 0.05) in percentage of surface covered in
S. Agona,
S. Enteritidis,
S. Infantis,
S. Thompson, and
S. 1,4,(5),12:i:- (
Table 6,
Figure 2). This treatment also caused a marked growth in the maximum thickness of the biofilm in the case of
S. Agona,
S. Enteritidis,
S. Infantis, and
S. 1,4,(5),12:i-, while the maximum thickness of the film diminished in strains of the serotypes
S. Anatum and
S. Typhimurium (
Table 7,
Figure 2).
An increased ability to form biofilm in the presence of low doses of SHY or BZK has been demonstrated previously for strains of
Escherichia coli [
34], methicillin-resistant
Staphylococcus aureus (MRSA) [
6],
Salmonella [
27], and
Listeria monocytogenes [
35]. In the present study the biofilms were exposed to disinfectants for ten minutes, and the biocides were then eliminated, even though residual quantities probably remained in the wells of the microtiter plate. A period of two to four hours elapsed between treatment with biocides and microscopic observation, a period during which the exposed strains (in contact with residual amounts of biocides) were able to synthesize biofilm to a greater extent than unexposed strains. The explanation for this greater production of biofilm by the strains exposed to residual doses of SHY or BZK may have to do with the adaptational response of the bacteria, appearing as changes to the structure, composition, or speed of growth of the bacterial cells or as an increase in the extracellular polymer matrix. The part that may be played by certain specialized cellular structures (for example, fimbriae and pili) in the augmented capacity of bacteria to form biofilm has also been highlighted [
6]. Nonetheless, further studies would be necessary to confirm these hypotheses.
BZK is usually employed at a dosage of 1000 to 5000 ppm [
9,
36,
37]. The increase in the biomass of biofilms after exposure for ten minutes to BZK at 1000 ppm underlines the need to further research into practical applications (i.e., on surfaces and equipment present in food-processing facilities) to substantiate these findings and to determine whether the recommended concentrations of BZK should be revised. This is especially crucial in the case of Gram-negative bacteria, which normally are more resistant to quaternary ammonium products as a consequence of modifications in the permeability of their cell walls or through an increased expression of unspecific efflux pumps [
38,
39].
At concentrations of 5000 and 10,000 ppm, BZK was effective in reducing the biovolume of the biofilms formed by most of the strains of
Salmonella (
Table 5). Exposure to BZK at 5000 ppm reduced the percentage of surface covered relative to the control strains in all cases, with the exception of
S. Enteritidis and
S. 1,4,(5),12:i:-. At 10,000 ppm, BZK was efficacious in decreasing the percentage of surface covered by the biofilms of all the strains (
Table 6). At 5000 and 10,000 ppm of BZK, the maximum thickness of the biofilms decreased in the case of
S. Anatum,
S. Hadar (10,000 ppm),
S. Kentucky, and
S. Typhimurium (
Table 7). These observations coincide with those in studies undertaken by other researchers, who have noted that the biocides used in the food industry are variable in their effects, which go from virtually total elimination of
Salmonella cells down to a very slight or almost zero reduction, depending on a series of factors, among which the concentrations at which they are used is prominent [
40].
Considerable differences were observed in the behavior of the various serotypes in respect to the surface roughness, or rugosity, of the biofilms after exposure to the disinfectants tested. After treatment with SHY, surface roughness tended to decrease in comparison to the unexposed biofilms in the cases of
S. Anatum,
S. Enteritidis,
S. Hadar,
S. Infantis, and
S. 1,4,(5),12:i:-. However, an increase in roughness was observed for biofilms of
S. Virchow after treatment with 25,000 ppm of SHY and for biofilms of
S. Agona and
S. Kentucky after treatment with 100,000 ppm of SHY (
Table 4). When the biofilms were exposed to 1000 ppm of BZK, rugosity of biofilms formed by
S. Anatum,
S. Enteritidis,
S. Hadar,
S. Thompson, and
S. 1,4,(5),12:1- decreased. After treatment with BZK at 5000 ppm or 10,000 ppm, roughness increased in the cases of the serotypes
S. Agona (10,000 ppm),
S. Anatum (10,000 ppm),
S. Kentucky (10,000 ppm),
S. Typhimurium, and
S. Virchow. BZK at 5000 ppm decreased roughness in
S. Anatum and
S. 1,4,(5),12:1- biofilms. In the other serotypes investigated, no variations were seen in the rugosity of biofilms (
Table 8).