Advances in Legionella Control by a New Formulation of Hydrogen Peroxide and Silver Salts in a Hospital Hot Water Network
Abstract
:1. Introduction
2. Results
2.1. Legionella Contamination
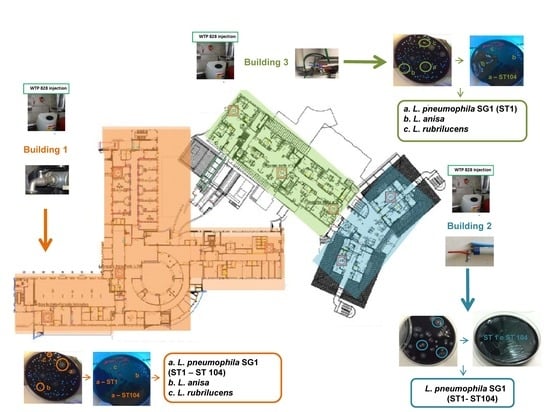
2.2. Legionella Typing
2.3. Pseudomonas aeruginosa and HPC Typing
2.4. Physical and Chemical Parameters of Water
2.5. LD Surveillance
3. Discussion
- -
- Building 1: WTP1 from October 2013 to December 2014 and WTP2 from January 2015 to October 2015;
- -
- Building 2: WTP1 from October 2013 to August 2014 and WTP2 from September 2014 to October 2015. This building was not subjected to any changes in disinfectant concentration or renovation works;
- -
- Building 3: WTP1 October 2013 to March 2015 and WTP2 from April 2015 to October 2015.
4. Materials and Methods
4.1. MCH Structure and Water Outlet Characteristics
- Building 1 covers an area of 18,539.93 m2 and has six floors with communal areas for the guests (e.g., bar, restrooms), operating rooms, outpatient services (diagnostic and consulting rooms), intensive care units, and 27 in-patient rooms located on the second floor. In this building, 21 sampling points and one hot water return line point were identified. Two of the 21 sampling points in in-patient rooms were monitored monthly, on a rotational basis by room number (Supplementary Table S1).
- Building 2 covers an area of 8178.68 m2, with six floors with 70 in-patient rooms distributed on floors one to four. Twenty-two sampling points were monitored (21 plus one hot water return line point) in this building, ten of which were in in-patient rooms, which were monitored monthly and rotated by room number (Supplementary Table S1).
- Building 3 covers an area of 1271.06 m2 and was recently expanded, with a complete renovation in February 2015. The building has six floors, with 25 in-patient rooms located on the third and fourth floors. Due to their size and comfort, these rooms are designated as “suites” and are reserved for long-term guests. There were 13 sampling points (and one hot water return line point) in this building, six of which were in in-patient rooms, which were monitored monthly and rotated by room number (Supplementary Table S1).
4.2. Hospital Water Network (HWN)
4.3. WTP 828
4.4. Study Design
- Building 1: WTP1 from October 2013 to December 2014 and WTP2 from January 2015 to October 2015;
- Building 2: WTP1 from October 2013 to August 2014 and WTP2 from September 2014 to October 2015. This building was not subjected to any changes in disinfectant concentration or renovation works;
- Building 3: WTP1 October 2013 to March 2015 and WTP2 from April 2015 to October 2015.
4.5. Sample Collection and Microbiological Analysis
4.6. Legionella Typing
4.7. Physical and Chemical Parameters of Water
4.8. Data Analyses
4.9. Hospital LD Surveillance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics Approval and Consent to Participate
Consent for Publication
Availability of Data Materials
References
- Hoffman, P.; Friedman, H.; Bendinelli, M. Legionella Pneumophila: Pathogenesis and Immunity; Springer: Berlin/Heidelberg, Germany, 2008; Volume XVIII, p. 208. [Google Scholar] [CrossRef]
- Cervero-Aragó, S.; Rodríguez-Martínez, S.; Puertas-Bennasar, A.; Araujo, R.M. Effect of Common Drinking Water Disinfectants, Chlorine and Heat, on Free Legionella and Amoebae-Associated Legionella. PLoS ONE 2015, 10, e0134726. [Google Scholar] [CrossRef]
- Blanc, D.; Carrara, P.; Zanetti, G.; Francioli, P.; Blanc, D. Water disinfection with ozone, copper and silver ions, and temperature increase to control Legionella: Seven years of experience in a university teaching hospital. J. Hosp. Infect. 2005, 60, 69–72. [Google Scholar] [CrossRef]
- Canals, O.; Serrano-Suárez, A.; Salvadó, H.; Méndez, J.; Cervero-Aragó, S.; Ruiz de Porras, V.; Dellundè, J.; Araujo, R. Effect of chlorine and temperature on free-living protozoa in operational man-made water systems (cooling towers and hot sanitary water systems) in Catalonia. Environ. Sci. Pollut. Res. Int. 2015, 22, 6610–6618. [Google Scholar] [CrossRef]
- Ministerial Decree 15 December 1990. Information System of Infectious and Diffusive Diseases (G.U. n.6 of 8 January 1991. Available online: http://www.salute.gov.it/imgs/C17normativa1357 allegato.pdf (accessed on 27 June 2018).
- Rota, M.C.; Caporali, M.G.; Bella, A.; Scaturro, M.; Giannitelli, S.; Ricci, M.L. Rapporto Annuale Sulla Legionellosi in Italia Nel 2017. Notiziario Ist. Super Sanità n.9, Volume 31. September 2018. Available online: http://www.legionellaonline.it/notiziario%20legionellosi%20_9_2018.pdf (accessed on 20 September 2019).
- Italian Health Ministry. Guidelines for Prevention and Control of Legionellosis. Approvate in Conferenza Stato-Regioni Seduta Del 7 Maggio 2015. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2362_allegato.pdf (accessed on 26 June 2018).
- Montagna, M.T.; De Giglio, O.; Napoli, C.; Diella, G.; Rutigliano, S.; Agodi, A.; Auxilia, F.; Baldovin, T.; Bisetto, F.; Arnoldo, L.; et al. Control and prevention measures for legionellosis in hospitals: A cross-sectional survey in Italy. Environ. Res. 2018, 166, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Anderson, J.; Mueller, S.; Gaines, W.; Kendall, A. Literature review—Efficacy of various disinfectants against Legionella in water systems. Water Res. 2002, 36, 4433–4444. [Google Scholar] [CrossRef]
- Borella, P.; Bargellini, A.; Marchegiano, P.; Vecchi, E.; Marchesi, I. Hospital-acquired Legionella infections: An update on the procedures for controlling environmental contamination. Ann. Ig. 2016, 28, 98–108. [Google Scholar] [PubMed]
- Farhat, M.; Moletta-Denat, M.; Frère, J.; Onillon, S.; Trouilhé, M.C.; Robine, E. Effects of disinfection on Legionella spp., eukarya, and biofilms in a hot water system. Appl. Environ. Microbiol. 2012, 78, 6850–6858. [Google Scholar] [CrossRef]
- Cristino, S.; Legnani, P.P.; Leoni, E. Plan for the control of Legionella infections in long-term care facilities: Role of environmental monitoring. Int. J. Hyg. Environ. Health 2012, 215, 279–285. [Google Scholar] [CrossRef]
- Bédard, E.; Boppe, I.; Kouamé, S.; Martin, P.; Pinsonneault, L.; Valiquette, L.; Racine, J.; Prévost, M. Combination of Heat Shock and Enhanced Thermal Regime to Control the Growth of a Persistent Legionella pneumophila Strain. Pathogens 2016, 5, 35. [Google Scholar] [CrossRef]
- White, G.C. The Handbook of Chlorination and Alternative Disinfectants, 4th ed.; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Florentin, A.; Hautemanière, A.; Hartemann, P. Health effects of disinfection by-products in chlorinated swimming pools. Int. J. Hyg. Environ. Health 2011, 214, 461–469. [Google Scholar] [CrossRef]
- Lin, Y.E.; Stout, J.E.; Yu, V.L. Controlling Legionella in Hospital Drinking Water: An Evidence-Based Review of Disinfection Methods. Infect. Control Hosp. Epidemiol. 2011, 32, 166–173. [Google Scholar] [CrossRef] [PubMed]
- WHO. Trihalomethanes in Drinking-Water. 2005. Available online: http://www.who.int/water_sanitation_health/dwq/chemicals/THM200605.pdf (accessed on 22 June 2018).
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; DeMarini, D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Plewa, M.J.; Lindell, C.L.; Richardson, S.D.; Mitch, W.A. Comparison of Byproduct Formation in Waters Treated with Chlorine and Iodine: Relevance to Point-of-Use Treatment. Environ. Sci. Technol. 2010, 44, 8446–8452. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Water Safety in Distribution Systems. WHO Library Cataloguing-in-Publication Data. 2014. Available online: http://www.who.int/water_sanitation_health/publications/WSH-distribution_system-20141114.pdf (accessed on 25 June 2018).
- Schoenen, D. Role of disinfection in suppressing the spread of pathogens with drinking water: Possibilities and limitations. Water Res. 2002, 36, 3874–3888. [Google Scholar] [CrossRef]
- Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.-Y. Use of hydrogen peroxide as a biocide: New consideration of its mechanisms of biocidal action. J. Antimicrob. Chemother. 2012, 67, 1589–1596. [Google Scholar] [CrossRef]
- Baldry, M. The bactericidal, fungicidal and sporicidal properties of hydrogen peroxide and peracetic acid. J. Appl. Bacteriol. 1983, 54, 417–423. [Google Scholar] [CrossRef]
- Martin, N.L.; Bass, P.; Liss, S.N. Antibacterial Properties and Mechanism of Activity of a Novel Silver-Stabilized Hydrogen Peroxide. PLoS ONE 2015, 10, e0131345. [Google Scholar] [CrossRef]
- Ricci, M.; Dell’Eva, I.; Scaturro, M.; Baruchelli, P.; De Ponte, G.; Losardo, M.; Ottaviani, M.; Guizzardi, F. Six-Month Experience of Silver-Hydrogen Peroxide Treatment for Legionella Control in Two Nursing Home Water Systems. In Legionella. State of the Art 30 Years after Its Recognition; Cianciotto, N., Kwaik, Y., Edelstein, P., Fields, B., Geary, D., Harrison, T., Joseph, C., Ratcliff, R., Stout, J., Swanson, M., Eds.; ASM Press: Washington, DC, USA, 2006; pp. 505–508. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 2001, 14, 227. [Google Scholar] [CrossRef]
- Regulation (EU) 528/2012 of the European Parliament and of the Council of 22 May 2012 Concerning the Making Available on the Market and Use of Biocide Products. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32012R0528&from=IT (accessed on 28 June 2018).
- Juven, B.J.; Pierson, M.D. Antibacterial Effects of Hydrogen Peroxide and Methods for Its Detection and Quantitation. J. Food Prot. 1996, 59, 1233–1241. [Google Scholar] [CrossRef]
- Slawson, R.M.; Van Dyke, M.I.; Lee, H.; Trevors, J.T. Germanium and silver resistance, accumulation, and toxicity in microorganisms. Plasmid 1992, 27, 72–79. [Google Scholar] [CrossRef]
- Loo, S.-L.; Fane, A.G.; Lim, T.-T.; Krantz, W.B.; Liang, Y.-N.; Liu, X.; Hu, X. Superabsorbent Cryogels Decorated with Silver Nanoparticles as a Novel Water Technology for Point-of-Use Disinfection. Environ. Sci. Technol. 2013, 47, 9363–9371. [Google Scholar] [CrossRef] [PubMed]
- Ehdaie, B.; Krause, C.; Smith, J.A. Porous Ceramic Tablet Embedded with Silver Nanopatches for Low-Cost Point-of-Use Water Purification. Environ. Sci. Technol. 2014, 48, 13901–13908. [Google Scholar] [CrossRef] [PubMed]
- Tugulea, A.-M.; Berube, D.; Giddings, M.; Lemieux, F.; Hnatiw, J.; Priem, J.; Avramescu, M.-L. Nano-silver in drinking water and drinking water sources: Stability and influences on disinfection by-product formation. Environ. Sci. Pollut. Res. 2014, 21, 11823–11831. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality. 2011. Available online: http://apps.who.int/iris/bitstream/10665/44584/1/9789241548151_eng.pdf (accessed on 28 June 2018).
- Pedahzur, R.; Lev, O.; Fattal, B.; Shuval, H.I. The interaction of silver ions and hydrogen peroxide in the inactivation of E. coli: A preliminary evaluation of a new long acting residual drinking water disinfectant. Water Sci. Technol. 1995, 31, 123–129. [Google Scholar] [CrossRef]
- Pedahzur, R. Silver and hydrogen peroxide as potential drinking water disinfectants: Their bactericidal effects and possible modes of action. Water Sci. Technol. 1997, 35, 87–93. [Google Scholar] [CrossRef]
- Nabizadeh, R.; Samadi, N.; Sadeghpour, Z.; Beikzadeh, M. Feasibility study of using complex of hydrogen peroxide and silver for disinfecting swimming pool water and its environment. Iran. J. Environ. Health 2008, 5, 235–242. [Google Scholar]
- Absalan, A.; Ehrampoush, M.; Davoudi, M.; Vakili, T.; Ebrahimi, A. Antibacterial effects of hydrogen peroxide and silver composition on selected pathogenic enterobacteriaceae. Int. J. Environ. Health Eng. 2012, 1, 23. [Google Scholar] [CrossRef]
- Thomas, V.; Bouchez, T.; Nicolas, V.; Robert, S.; Loret, J.; Levi, Y. Amoebae in domestic water systems: Resistance to disinfection treatments and implication in Legionella persistence. J. Appl. Microbiol. 2004, 97, 950–963. [Google Scholar] [CrossRef]
- Berry, D.; Xi, C.; Raskin, L. Microbial ecology of drinking water distribution systems. Curr. Opin. Biotechnol. 2006, 17, 297–302. [Google Scholar] [CrossRef]
- Ditommaso, S.; Giacomuzzi, M.; Ricciardi, E.; Zotti, C.M. Efficacy of a Low Dose of Hydrogen Peroxide (Peroxy Ag+) for Continuous Treatment of Dental Unit Water Lines: Challenge Test with Legionella pneumophila Serogroup 1 in a Simulated Dental Unit Waterline. Int. J. Environ. Res. Public Health 2016, 13, 745. [Google Scholar] [CrossRef]
- Shuval, H.; Shenman, R.; Yarom, R. An Innovative Method for the Control of Legionella Infections in the Hospital Hot Water Systems with a Stabilized Hydrogen Peroxide-Silver Formulation. Int. J. Infect. Control 2009, 5. [Google Scholar] [CrossRef]
- Casini, B.; Aquino, F.; Totaro, M.; Miccoli, M.; Galli, I.; Manfredini, L.; Giustarini, C.; Costa, A.L.; Tuvo, B.; Valentini, P.; et al. Application of Hydrogen Peroxide as an Innovative Method of Treatment for Legionella Control in a Hospital Water Network. Pathogens 2017, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Rasamiravaka, T.; Labtani, Q.; Duez, P.; El Jaziri, M. The Formation of Biofilms by Pseudomonas aeruginosa: A Review of the Natural and Synthetic Compounds Interfering with Control Mechanisms. BioMed Res. Int. 2015, 2015, 759348. [Google Scholar] [CrossRef] [PubMed]
- Duda, S.; Baron, J.L.; Wagener, M.M.; Vidic, R.D.; Stout, J.E. Lack of correlation between Legionella colonization and microbial population quantification using heterotrophic plate count and adenosine triphosphate bioluminescence measurement. Environ. Monit. Assess. 2015, 187, 393. [Google Scholar] [CrossRef]
- Commission Regulation (EC) 1451/2007. Available online: http://eur-lex.europa.eu/legal-content/IT/TXT/PDF/?uri=CELEX:32007R1451&from=it (accessed on 28 June 2018).
- Decreto Legislativo 2 febbraio 2001, n. 31, Attuazione Della Direttiva 98/83/CE Relativa Alla Qualità Delle Acque Destinate al Consumo Umano (G.U. n. 52 Del 3 Marzo 2001—s.o.n. 41). Available online: http://www.gazzettaufficiale.it/eli/id/2001/03/03/001G0074/sg (accessed on 22 June 2018).
- Marchesi, I.; Ferranti, G.; Mansi, A.; Marcelloni, A.M.; Proietto, A.R.; Saini, N.; Borella, P.; Bargellini, A. Control of Legionella Contamination and Risk of Corrosion in Hospital Water Networks following Various Disinfection Procedures. Appl. Environ. Microbiol. 2016, 82, 2959–2965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahapatra, A.; Padhi, N.; Mahapatra, D.; Bhatt, M.; Sahoo, D.; Jena, S.; Dash, D.; Chayani, N. Study of Biofilm in Bacteria from Water Pipelines. J. Clin. Diagn. Res. 2015, 9, DC09–DC11. [Google Scholar] [CrossRef]
- Sabria, M.; Yu, V.L. Hospital-acquired legionellosis: Solutions for a preventable infection. Lancet Infect. Dis. 2002, 2, 368–373. [Google Scholar] [CrossRef]
- Douterelo, I.; Sharpe, R.; Boxall, J. Influence of hydraulic regimes on bacterial community structure and composition in an experimental drinking water distribution system. Water Res. 2013, 47, 503–516. [Google Scholar] [CrossRef]
- Liu, S.; Gunawan, C.; Barraud, N.; Rice, S.A.; Harry, E.J.; Amal, R. Understanding, Monitoring, and Controlling Biofilm Growth in Drinking Water Distribution Systems. Environ. Sci. Technol. 2016, 50, 8954–8976. [Google Scholar] [CrossRef]
- Rogers, J.; Dowsett, A.B.; Dennis, P.J.; Lee, J.V.; Keevil, C.W. Influence of Plumbing Materials on Biofilm Formation and Growth of Legionella pneumophila in Potable Water Systems. Appl. Environ. Microbiol. 1994, 60, 1842–1851. [Google Scholar]
- Lehtola, M.J.; Laxander, M.; Miettinen, I.T.; Hirvonen, A.; Vartiainen, T.; Martikainen, P.J. The effects of changing water flow velocity on the formation of biofilms and water quality in pilot distribution system consisting of copper or polyethylene pipes. Water Res. 2006, 40, 2151–2160. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Kim, D.; Lee, T. Microbial diversity in biofilms on water distribution pipes of different materials. Water Sci. Technol. 2010, 61, 163–171. [Google Scholar] [CrossRef]
- Rożej, A.; Cydzik-Kwiatkowska, A.; Kowalska, B.; Kowalski, D. Structure and microbial diversity of biofilms on different pipe materials of a model drinking water distribution systems. World J. Microbiol. Biotechnol. 2015, 31, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Schwake, D.O.; Alum, A.; Abbaszadegan, M. Impact of Environmental Factors on Legionella Populations in Drinking Water. Pathogens 2015, 4, 269–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wingender, J.; Flemming, H.-C. Contamination potential of drinking water distribution network biofilms. Water Sci. Technol. 2004, 49, 277–286. [Google Scholar] [CrossRef]
- Fields, B.S.; Benson, R.F.; Besser, R.E. Legionella and Legionnaires’ Disease: 25 Years of Investigation. Clin. Microbiol. Rev. 2002, 15, 506–526. [Google Scholar] [CrossRef]
- Bartram, J.; Chartlier, Y.; Lee, J.V.; Pond, K.; Surman-Lee, S. Legionella and the Prevention of Legionellosis; World Health Organization: Geneva, Switzerland, 2007; Available online: http://www.who.int/water_sanitation_health/emerging/legionella.pdf (accessed on 21 June 2018).
- Brenner, D.J.; Steigerwalt, A.G.; Gorman, G.W.; Wilkinson, H.W.; Bibb, W.F.; Hackel, M.; Tyndall, R.L.; Campbell, J.; Feeley, J.C.; Thacker, W.L.; et al. Ten new species of Legionella. Int. J. Syst. Evol. Microbiol. 1985, 35, 50–59. [Google Scholar] [CrossRef]
- Casati, S.; Conza, L.; Bruin, J.; Gaia, V. Compost facilities as a reservoir of Legionella pneumophila and other Legionella species. Clin. Microbiol. Infect. 2010, 16, 945–947. [Google Scholar] [CrossRef] [Green Version]
- Duda, S.; Kandiah, S.; Stout, J.E.; Baron, J.L.; Yassin, M.; Fabrizio, M.; Ferrelli, J.; Hariri, R.; Wagener, M.M.; Goepfert, J.; et al. Evaluation of a New Monochloramine Generation System for Controlling Legionella in Building Hot Water Systems. Infect. Control Hosp. Epidemiol. 2014, 35, 1356–1363. [Google Scholar] [CrossRef]
- Husband, P.; Boxall, J. Asset deterioration and discolouration in water distribution systems. Water Res. 2011, 45, 113–124. [Google Scholar] [CrossRef]
- El-Chakhtoura, J.; Saikaly, P.E.; Van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Impact of Distribution and Network Flushing on the Drinking Water Microbiome. Front. Microbiol. 2018, 9, 2205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Council of the European Union. European Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption. Off. J. Eur. Community 1998, 330, 32–54. [Google Scholar]
- Imlay, J.A. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat. Rev. Genet. 2013, 11, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Vatansever, F.; De Melo, W.C.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered around reactive oxygen species—Bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef]
- Pedahzur, R.; Katzenelson, D.; Barnea, N.; Lev, O.; Shuval, H.; Fattal, B.; Ulitzur, S. The efficacy of long-lasting residual drinking water disinfectants based on hydrogen peroxide and silver. Water Sci. Technol. 2000, 42, 293–298. [Google Scholar] [CrossRef]
- ISO 11731:2017 Water Quality—Enumeration of Legionella. Available online: https://www.iso.org/standard/61782.html (accessed on 22 June 2018).
- Kimura, S.; Tateda, K.; Ishii, Y.; Horikawa, M.; Miyairi, S.; Gotoh, N.; Ishiguro, M.; Yamaguchi, K. Pseudomonas aeruginosa Las quorum sensing autoinducer suppresses growth and biofilm production in Legionella species. Microbiology 2009, 155, 1934–1939. [Google Scholar] [CrossRef] [Green Version]
- UNI EN ISO 16266:2006 Water Quality—Detection and Enumeration of Pseudomonas Aeruginosa—Method by Membrane Filtration. 2006. Available online: https://www.iso.org/standard/39272.html (accessed on 24 June 2018).
- National Research Council. Drinking Water Distribution Systems: Assessing and Reducing Risks; The National Academies Press: Washington, DC, USA, 2006. [CrossRef]
- UNI EN ISO 6222:2001 Water Quality—Enumeration of Culturable Micro-Organisms—Colony Count by Inoculation in a Nutrient Agar Culture Medium. 2001. Available online: https://www.iso.org/standard/28960.html (accessed on 24 June 2018).
- Ratcliff, R.M.; Lanser, J.A.; Manning, P.A.; Heuzenroeder, M.W. Sequence-Based Classification Scheme for the Genus Legionella Targeting the mip Gene. J. Clin. Microbiol. 1998, 36, 1560–1567. [Google Scholar]
- Mentasti, M.; Fry, N.K.; Afshar, B.; Palepou-Foxley, C.; Naik, F.C.; Harrison, T.G.; Fry, N. Application of Legionella pneumophila-specific quantitative real-time PCR combined with direct amplification and sequence-based typing in the diagnosis and epidemiological investigation of Legionnaires’ disease. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2017–2028. [Google Scholar] [CrossRef]
- Fry, N.K.; Alexiou-Daniel, S.; Bangsborg, J.M.; Bernander, S.; Castellani Pastoris, M.; Etienne, J.; Forsblom, B.; Gaia, V.; Helbig, J.H.; Lindsay, D.; et al. A multicenter evaluation of genotypic methods for the epidemiological typing of Legionella pneumophila serogroup 1: Results of a pan-European study. Clin. Microbiol. Infec. 1999, 5, 462–477. [Google Scholar] [CrossRef]
- Fry, N.; Afshar, B.; Bellamy, W.; Underwood, A.P.; Ratcliff, R.; Harrison, T.G. Identification of Legionella spp. by 19 European reference laboratories: Results of the European Working Group for Legionella Infections External Quality Assessment Scheme using DNA sequencing of the macrophage infectivity potentiator gene and dedicated online tools. Clin. Microbiol. Infect. 2007, 13, 1119–1124. [Google Scholar]
- APAT. Agenzia per la Protezione Dell’ambiente e per i Servizi Tecnici. Manuali e Linee Guida 29/2003: Metodi Analitici per le Acque. 2003. Available online: http://www.isprambiente.gov.it/it/pubblicazioni/manuali-e-linee-guida/metodi-analitici-per-le-acque (accessed on 25 June 2018).

| Treatment/Study Phase | Number of Samples | Number of Legionella Positive Samples (%) | Mean Legionella Levels (log10 CFU/L) ± SD | Comparison between Phases | p Value | |
|---|---|---|---|---|---|---|
| MCH (Buildings 1–3) | ClO2 mixture | 120 | 114 (95.0) | 2.54 ± 0.74 | WTP1 vs. ClO2 | 1 |
| WTP1 | 53 | 32 (60.0) | 2.43 ± 0.95 | WTP1 vs. WTP2 | 0.0001 * | |
| WTP2 | 296 | 106 (35.8) | 1.67 ± 0.66 | WTP2 vs. ClO2 | 0.0001 * | |
| Building 1 | ClO2 mixture | 47 | 46 (98.0) | 2.47 ± 0.67 | WTP1 vs. ClO2 | 0.623 |
| WTP1 | 25 | 16 (64.0) | 2.80 ± 0.87 | WTP1 vs. WTP2 | 0.060 | |
| WTP2 | 141 | 82 (58.1) | 2.21 ± 0.55 | WTP2 vs. ClO2 | 0.835 | |
| Building 2 | ClO2 mixture | 58 | 53 (91.3) | 2.39 ± 0.63 | WTP1 vs. ClO2 | 0.045 * |
| WTP1 | 23 | 13 (56.5) | 1.81 ± 0.82 | WTP1 vs. WTP2 | 0.046 * | |
| WTP2 | 108 | 8 (7.0) | 1.26 ± 0.40 | WTP2 vs. ClO2 | 0.0001 * | |
| Building 3 | ClO2 mixture | 15 | 15 (100.0) | 3.12 ± 1.04 | WTP1 vs. ClO2 | 1 |
| WTP1 | 5 | 3 (60.0) | 2.97 ± 0.71 | WTP1 vs. WTP2 | 0.048 * | |
| WTP2 | 47 | 16 (34.0) | 1.47 ± 0.60 | WTP2 vs. ClO2 | 0.01 * |
| Study phases | Odds Ratio (OR) | 95% Confidence Intervals | p value |
| ClO2 mixture versus WTP1 | 0.30 | 0.09–1.02 | 0.048 * |
| ClO2 mixture versus WTP2 | 15.44 | 5.14–46.33 | 0.0001 * |
| Study phases | Relative Risk (RR) | 95% Confidence Intervals | p value |
| WTP1 versus WTP2 | 0.36 | 0.18–0.71 | 0.002 * |
| Building | Positive Samples | Serotyping | Genotyping | Legionella Isolates WTP1 Versus WTP2 | Isolates/Positive Samples | Range of Legionella Concentration log10 CFU/L (Min–Max) * | |
|---|---|---|---|---|---|---|---|
| Building 1 | 98 | L. pneumophila SG1 | ST1 and ST104 | WTP1 | 36/98 | <1.70–5.80 | |
| L. species | L. rubrilucens | WTP2 | 11/98 | <1.70–4.60 | |||
| L. species | L. anisa | WTP2 | 20/98 | <1.70–3.77 | |||
| L. species | L. rubrilucens + L. anisa | WTP2 | 2/98 | 2.83–3 | |||
| L. pneumophila SG1 + L. species | ST1 and ST104 + L. rubrilucens | WTP2 | 29/98 | 9/29 | 2–5.69 | ||
| ST1 and ST104 + L. anisa | WTP2 | 20/29 | <1.70–3.53 | ||||
| Building 2 | 21 | L. pneumophila SG1 | ST1 and ST104 | WTP1 and WTP2 | 21/21 | <1.70–4.50 | |
| Building 3 | 19 | L. pneumophila SG1 | ST1 | WTP1 | 17/19 | <1.70–4.18 | |
| L. species | L. rubrilucens | WTP2 | 2/19 | 1/19 | 2.10 | ||
| L. anisa | WTP2 | 1/19 | 1.70 | ||||
| Parameters | Standardized Methods | Principle of the Method | U.M. | Sampling Points (Mean Value ± SD) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aqueduct (n = 13) | Building 1 | Building 2 | Building 3 | |||||||
| Hot Water Return Line (1a) * (n = 13) | Distal Outlets (n = 128) | Hot Water Return Line (1b) * (n = 13) | Distal Outlets (n = 95) | Hot Water Return Line (1c) * (n = 3) | Distal Outlets (n = 34) | |||||
| pH | APAT IRSA CNR 2060 Man 29 2003 | Potentiometric method | 7.82 ± 0.23 | 7.88 ± 0.24 | 7.95 ± 0.07 | 7.87 ± 0.27 | 7.92 ± 0.04 | 7.95 ± 0.19 | 7.94 ± 0.09 | |
| Hardness | APAT IRSA CNR 2040 Man 29 2003 | Complex metric titration | ° f | 12.10 ± 4.28 | 12.13 ± 3.14 | 11.07 ± 3.46 | 12.63 ± 3.50 | 9.75 ± 4.44 | 12.15 ± 2.44 | 11.92 ± 3.05 |
| Conductivity | APAT IRSA CNR 2030 Man 29 2003 | Conductimetric method | µS/cm | 407.71 ± 35.20 | 416.53 ± 41.80 | 374.91 ± 39.97 | 420.00 ± 40.70 | 447 ± 13.78 | 423.44 ± 29.75 | 424.80 ± 32.09 |
| Turbidity | APAT IRSA CNR 2110 Man 29 2003 | Spectrophotometric method | NTU | 0.40 ± 0.09 | 0.39 ± 0.12 | 0.63 ± 0.22 | 0.52 ± 0.25 | 1.69 ± 1.41 | 0.88 ± 0.97 | 0.94 ± 0.63 |
| Total iron | APAT IRSA CNR 3160A Man 29 2003 | Flame atomic absorption spectroscopy (FAAS) | mg/L | <0.04 | 0.04 | 0.05 ± 0.03 | <0.04 | <0.03 | 0.03 | 0.09 ± 0.10 |
| Total phosphorus | APAT IRSA CNR 4060 Man 29 2003 | Spectrophotometric method | mg/L P2O5 | <0.2 | 3.19 ± 1.31 | 3.05 ± 0.49 | 3.40 ± 1.40 | 3.52 ± 0.76 | 1.68 ± 0.85 | 2.05 ± 0.59 |
| Silver | EPA Method 272.2 | Electrothermal atomization atomic absorption spectrometry (ETA-AAS) | µg/L | <3 | <3 | <3 | <3 | <3 | <3 | <3 |
| Temperature | EPA Method 170.1 | Thermistor probe | °C | 15.20 ± 3.40 | 49.03 ± 2.42 | 49.75 ± 2.30 | 50.20 ± 0.61 | 50.38 ± 1.80 | 53.87 ± 4.13 | 51.07 ± 2.25 |
| Peroxide | Peroxide Test MQuant ™ | mg/L | not detected | 7.42 ± 2.71 | 15.0 ± 7.07 | 8.46 ± 3.15 | 14.72 ± 4.75 | 5.83 ± 3.84 | 10 ± 4.63 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girolamini, L.; Dormi, A.; Pellati, T.; Somaroli, P.; Montanari, D.; Costa, A.; Savelli, F.; Martelli, A.; Grottola, A.; Fregni Serpini, G.; et al. Advances in Legionella Control by a New Formulation of Hydrogen Peroxide and Silver Salts in a Hospital Hot Water Network. Pathogens 2019, 8, 209. https://doi.org/10.3390/pathogens8040209
Girolamini L, Dormi A, Pellati T, Somaroli P, Montanari D, Costa A, Savelli F, Martelli A, Grottola A, Fregni Serpini G, et al. Advances in Legionella Control by a New Formulation of Hydrogen Peroxide and Silver Salts in a Hospital Hot Water Network. Pathogens. 2019; 8(4):209. https://doi.org/10.3390/pathogens8040209
Chicago/Turabian StyleGirolamini, Luna, Ada Dormi, Tiziana Pellati, Paolo Somaroli, Davide Montanari, Andrea Costa, Francesca Savelli, Andrea Martelli, Antonella Grottola, Giulia Fregni Serpini, and et al. 2019. "Advances in Legionella Control by a New Formulation of Hydrogen Peroxide and Silver Salts in a Hospital Hot Water Network" Pathogens 8, no. 4: 209. https://doi.org/10.3390/pathogens8040209
APA StyleGirolamini, L., Dormi, A., Pellati, T., Somaroli, P., Montanari, D., Costa, A., Savelli, F., Martelli, A., Grottola, A., Fregni Serpini, G., & Cristino, S. (2019). Advances in Legionella Control by a New Formulation of Hydrogen Peroxide and Silver Salts in a Hospital Hot Water Network. Pathogens, 8(4), 209. https://doi.org/10.3390/pathogens8040209