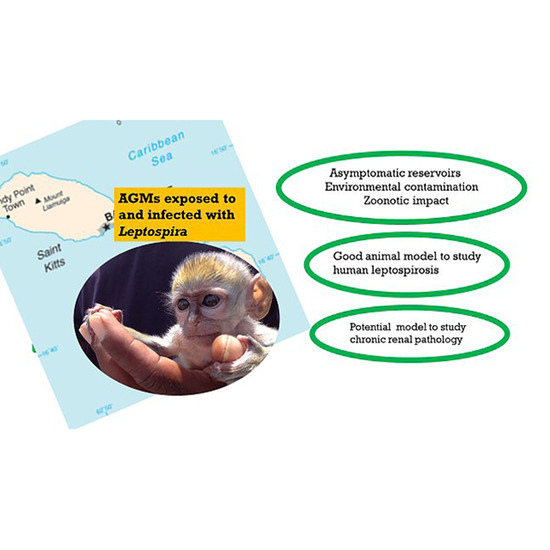
Leptospira Infection in African Green Monkeys in an Endemic Area: An Opportunity for Comparative Studies in a Natural Environment
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haake, D.A.; Levett, P.N. Leptospirosis in humans. Curr. Top. Microbiol. Immunol. 2015, 387, 65–97. [Google Scholar]
- Ellis, W.A. Animal Leptospirosis. Curr. Top. Microbiol. Immunol. 2015, 387, 99–137. [Google Scholar] [PubMed]
- Poingt, J.-P.; Perolat, P.; Jouaneau, C.; Gysin, J.; Vie, J.-C.; Baranton, G. Occurrence of Severe Leptospirosis in a Breeding Colony of Squirrel Monkeys. Am. J. Trop. Med. Hyg. 1992, 46, 538–545. [Google Scholar]
- Szonyi, B.; Agudelo-Flórez, P.; Ramírez, M.; Moreno, N.; Ko, A.I. An outbreak of severe leptospirosis in capuchin (Cebus) monkeys. Vet. J. 2011, 188, 237–239. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez Astudillo, V.; Wehdeking Hernandez, D.; Pena Stadlin, J.; Arias Bernal, L.; Lombo Rodriguez, D.A.; Astudillo Hernandez, M. comparative seroprevalence of leptospira interrogans in colombian mammals along a climatic gradient. J. Zoo Wildl. Med. 2012, 43, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Kinjo, T.; Minamoto, N.; Sugiyama, M.; Matsubayashi, N.; Narama, I. Prevalence Of Antibodies To Five Selected Zoonosis Agents In Monkeys. J. Vet. Med. Sci. 1991, 53, 553–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, R.B.; BAskerville, A.; Hambleton, P.; Adams, G.D.J. Benign leptospirosis: The pathology of experimental infection of monkeys with Leptospira interrogans serovars balcanica and tarassovi. Br. J. Exp. Pathol. 1980, 61, 124–131. [Google Scholar]
- Hambleton, P.; Baskerville, A.; Marshall, R.B.; Harris-Smith, P.W.; Adams, G.D. Metabolic sequelae of experimental leptospirosis in grivet monkeys. Br. J. Exp. Pathol. 1980, 61, 124–131. [Google Scholar]
- Marchevsky, R.S.; Lenzi, H.L.; Pinto, M.A.; Machado, M.P.; Da Silva, M.F.; Da Silva, J.J.P.; Pereira, M.M. Experimental Leptospirosis In Marmoset Monkeys (Callithrix jacchus): A New Model For Studies Of Severe Pulmonary Leptospirosis. Am. J. Trop. Med. Hyg. 2005, 72, 13–20. [Google Scholar]
- Rajeev, S.; Conan, A.; Pratt, N.; Beierschmitt, A.; Palmour, R. High Leptospira seroprevalence in captive and wild-caught vervet monkeys (Chlorocebus sabeus) on the Caribbean island of Saint Kitts. J. Vet. Diagn. Investig. 2017, 29, 930–934. [Google Scholar] [CrossRef] [Green Version]
- Costa, F.; Hagan, J.E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira, M.S.; Stein, C.; Abela-Ridder, B.; Ko, A.I. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl. Trop. Dis. 2015, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Shiokawa, K.; Llanes, A.; Hindoyan, A.; Cruz-Martinez, L.; Welcome, S.; Rajeev, S. Peridomestic small Indian mongoose: An invasive species posing as potential zoonotic risk for leptospirosis in the Caribbean. Acta Trop. 2019, 190, 166–170. [Google Scholar] [CrossRef]
- Rajeev, S.; Shiokawa, K.; Llanes, A.; Rajeev, M.; Restrepo, C.M.; Chin, R.; Cedeño, E.; Ellis, E. Detection and Characterization of Leptospira Infection and Exposure in Rats on the Caribbean Island of Saint Kitts. Animals 2020, 10, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiokawa, K.; Welcome, S.; Kenig, M.; Lim, B.; Rajeev, S. Epidemiology of Leptospira infection in livestock species in Saint Kitts. Trop. Anim. Health Prod. 2019, 51, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Larson, C.R.; Dennis, M.; Nair, R.V.; Llanes, A.; Peda, A.; Welcome, S.; Rajeev, S. Isolation and characterization of Leptospira interrogans serovar Copenhageni from a dog from Saint Kitts. JMM Case Rep. 2017, 4, e005120. [Google Scholar] [CrossRef] [Green Version]
- Pratt, N.; Conan, A.; Rajeev, S. Leptospira Seroprevalence in Domestic Dogs and Cats on the Caribbean Island of Saint Kitts. Vet. Med. Int. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [Green Version]
- McGuire, M.T. The St. Kitts vervet (Cercopithecus aethiops). J. Med. Primatol. 1974, 3, 287–295. [Google Scholar] [CrossRef]
- LaCroix, K.; Callanan, J.J.; Cruz-Martinez, L.; Rajeev, S. Prevalence of Renal Lesions in the Small Indian Mongoose (Herpestes auropunctatus) Inhabiting the Caribbean Island of Saint Kitts. J. Wildl. Dis. 2018, 54, 881–884. [Google Scholar] [CrossRef]
- Desai, S.; van Treeck, U.; Lierz, M.; Espelage, W.; Zota, L.; Sarbu, A.; Czerwinski, M.; Sadkowska-Todys, M.; Avdicová, M.; Reetz, J.; et al. Resurgence of Field Fever in a Temperate Country: An Epidemic of Leptospirosis among Seasonal Strawberry Harvesters in Germany in 2007. Clin. Infect. Dis. 2009, 48, 691–697. [Google Scholar] [CrossRef]
- Katelaris, A.L.; Glasgow, K.; Lawrence, K.; Corben, P.; Zheng, A.; Sumithra, S.; Turahui, J.; Terry, J.; van den Berg, D.; Hennessy, D.; et al. Investigation and response to an outbreak of leptospirosis among raspberry workers in Australia, 2018. Zoonoses Public Health 2019, 67, 35–43. [Google Scholar] [CrossRef]
- Levett, P.N. Usefulness of Serologic Analysis as a Predictor of the Infecting Serovar in Patients with Severe Leptospirosis. Clin. Infect. Dis. 2003, 36, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Hung, C.C.; Liu, S.H.; Guo, Y.G.; Chen, Y.C.; Ko, Y.C.; Huang, C.T.; Chou, L.F.; Tian, Y.C.; Chang, M.Y.; et al. Overlooked Risk for Chronic Kidney Disease after Leptospiral Infection: A Population-Based Survey and Epidemiological Cohort Evidence. PLoS Negl. Trop. Dis. 2015, 9, 1–15. [Google Scholar] [CrossRef]
- Yang, C.W. Leptospirosis Renal Disease: Emerging Culprit of Chronic Kidney Disease Unknown Etiology. Nephron 2017, 138, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Jasinska, A.J.; Schmitt, C.A.; Service, S.K.; Cantor, R.M.; Dewar, K.; Jentsch, J.D.; Kaplan, J.R.; Turner, T.R.; Warren, W.C.; Weinstock, G.M.; et al. Systems Biology of the Vervet Monkey. ILAR J. 2013, 54, 122–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goris, M.G.A.; Hartskeerl, R.A. Leptospirosis serodiagnosis by the microscopic agglutination test. Curr. Protoc. Microbiol. 2014, 32, 1–18. [Google Scholar]
- Stoddard, R.A. Detection of pathogenic leptospira spp. through real-time PCR (qPCR) targeting the lipL32 gene. Methods Mol. Biol. 2013, 943, 257–266. [Google Scholar]


| Species | Serogroup | Serovar (Results) |
|---|---|---|
| L. interrogans | Australis | Australis (Negative) |
| Bratislava (Positive) | ||
| Autumnalis | Autumnalis (Positive) | |
| Bataviae | Bataviae (Positive) | |
| Canicola | Canicola (Negative) | |
| Djasiman | Djasiman (Positive) | |
| Grippotyphosa | Grippotyphosa (Negative) | |
| Icterohemorrhagiae | Icterohemorrhagiae (Positive) | |
| Mankarso (Positive) | ||
| Copenhageni (Positive) | ||
| Pomona | Pomona (Positive) | |
| Sejroe | Hardjo (Negative) | |
| Wolffii (Negative) | ||
| L. borgpetersenii | Ballum | Ballum (Positive) |
| Javanica | Javanica (Negative) | |
| Tarrasovi | Tarrasovi (Negative) | |
| L. santarosai | Pyrogenes | Alexi (Negative) |
| Pyrogenes (Negative) | ||
| Mini | Georgia (positive) | |
| L. kirschneri | Cynopteri | Cynopteri (Positive) |
| L. weilii | Celledoni | Celledoni (Positive) |
| AGM # | Serovars Positive (MAT-Titer) |
|---|---|
| 3 | Autumnalis (200), Bratislava (1600) |
| 4 | Ballum (200) |
| 5 | Bataviae (100) |
| 7 | Ballum (100) |
| 10 | Ballum (400) |
| 14 | Ballum (50) |
| 15 | Ballum (50) |
| 32 | Djasiman(50) |
| 36 | Icterohemorrhagiae (200), Mankarso (50) |
| 37 | Icterohemorrhagiae (200), Mankarso(100), Copenhageni (100) |
| 38 | Bataviae (100) |
| 39 | Bataviae (100) |
| 40 | Icterohemorrhagiae (100) Mankarso(50) |
| 41 | Icterohemorrhagiae (50) |
| 42 | Bataviae (100) |
| 43 | Icterohemorrhagiae (200), Mankarso (100), Ballum (400), Bataviae (100) |
| 45 | Icterohemorrhagiae (50), Bataviae (100) |
| 46 | Icterohemorrhagiae (400), Mankarso (400), Copenhageni (200) |
| 47 | Bataviae (100) |
| 48 | Bataviae (200) |
| 51 | Bataviae (50) |
| 53 | Icterohemorrhagiae (50), Mankarso(50) |
| 55 | Bataviae (100) |
| 56 | Ballum (100) |
| 57 | Icterohemorrhagiae (400), Mankarso (800), Copenhageni (100) |
| 66 | Autumnalis (50) |
| 67 | Autumnalis (50) |
| 69 | Autumnalis (200), Cynopteri (50), Georgia (50), Icterohemorrhagiae (100), Mankarso (200), Copenhageni (100) |
| 70 | Bratislava (50) |
| 71 | Mankarso (100), Copenhageni (50) |
| 72 | Autumnalis (50), Mankarso (100) |
| 73 | Ballum (200), Bataviae (100),Celledoni (50), Mankarso (100) |
| 74 | Autumnalis (400), Bratislava (50), Pomona (50) |
| 76 | Ballum (100), Bataviae (100), Mankarso (50) |
| 77 | Bataviae (50) |
| 78 | Ballum (100) |
| 79 | Ballum (200), Bataviae (100) |
| 81 | Ballum (50) Mankarso (50), Copenhageni (50) |
| 83 | Bataviae (50), Djasiman (50) |
| ISN+ | ISN− | Total | |
|---|---|---|---|
| MAT+ | 7 | 1 | 8 |
| MAT− | 22 | 4 | 26 |
| Total | 29 | 5 | 34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajeev, S.; Bolfa, P.; Shiokawa, K.; Beierschmitt, A.; Palmour, R. Leptospira Infection in African Green Monkeys in an Endemic Area: An Opportunity for Comparative Studies in a Natural Environment. Pathogens 2020, 9, 474. https://doi.org/10.3390/pathogens9060474
Rajeev S, Bolfa P, Shiokawa K, Beierschmitt A, Palmour R. Leptospira Infection in African Green Monkeys in an Endemic Area: An Opportunity for Comparative Studies in a Natural Environment. Pathogens. 2020; 9(6):474. https://doi.org/10.3390/pathogens9060474
Chicago/Turabian StyleRajeev, Sreekumari, Pompei Bolfa, Kanae Shiokawa, Amy Beierschmitt, and Roberta Palmour. 2020. "Leptospira Infection in African Green Monkeys in an Endemic Area: An Opportunity for Comparative Studies in a Natural Environment" Pathogens 9, no. 6: 474. https://doi.org/10.3390/pathogens9060474
APA StyleRajeev, S., Bolfa, P., Shiokawa, K., Beierschmitt, A., & Palmour, R. (2020). Leptospira Infection in African Green Monkeys in an Endemic Area: An Opportunity for Comparative Studies in a Natural Environment. Pathogens, 9(6), 474. https://doi.org/10.3390/pathogens9060474