Using Microbiome-Based Approaches to Deprogram Chronic Disorders and Extend the Healthspan following Adverse Childhood Experiences
Abstract
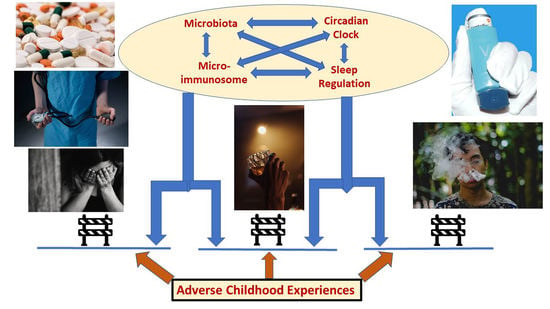
:1. Introduction
2. Adverse Childhood Experiences and the Microimmunosome
3. The Range of ACE-Programmed Chronic Diseases and Disorders
4. ACE-Programmed Misregulated Inflammation and Specific NCDs
5. Additional Outcomes of ACEs with Microbiota Regulation
5.1. Pain
5.2. Substance Misuse/Abuse
6. At the Epicenter of NCDs
7. Circadian Rhythms
8. Sleep and Microbiota
9. Inflammation, Oxidative Stress, and the Longevity Cycle
10. The Immunological Epigenetic Clock of the Microimmunosome
11. Gerobiotics as a Microbiota-Based Anti-Aging/Healthspan Strategy
12. Discussion
13. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barker, D.J. The fetal and infant origins of adult disease. BMJ 1990, 301, 1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, T.E.; Golemboski, K.A.; Ha, R.S.; Bunn, T.; Sanders, F.S.; Dietert, R.R. Developmental exposure to lead causes persistent immunotoxicity in Fischer 344 rats. Toxicol. Sci. 1998, 42, 129–135. [Google Scholar] [CrossRef]
- Dietert, R.R.; Etzel, R.A.; Chen, D.; Halonen, M.; Holladay, S.D.; Jarabek, A.M.; Landreth, K.; Peden, D.B.; Pinkerton, K.; Smialowicz, R.J.; et al. Workshop to identify critical windows of exposure for children’s health: Immune and respiratory systems work group summary. Environ. Health Perspect. 2000, 108, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Barouki, R.; Gluckman, P.D.; Grandjean, P.; Hanson, M.; Heindel, J.J. Developmental origins of non-communicable disease: Implications for research and public health. Environ. Health 2012, 11, 42. [Google Scholar] [CrossRef] [Green Version]
- Tewari, S.; Toledo Margalef, P.; Kareem, A.; Abdul-Hussein, A.; White, M.; Wazana, A.; Davidge, S.T.; Delrieux, C.; Connor, K.L. Mining Early Life Risk and Resiliency Factors and Their Influences in Human Populations from PubMed: A Machine Learning Approach to Discover DOHaD Evidence. J. Pers. Med. 2021, 11, 1064. [Google Scholar] [CrossRef] [PubMed]
- Aversa, Z.; Atkinson, E.J.; Schafer, M.J.; Theiler, R.N.; Rocca, W.A.; Blaser, M.J.; LeBrasseur, N.K. Association of Infant Antibiotic Exposure With Childhood Health Outcomes. Mayo Clin. Proc. 2021, 96, 66–77. [Google Scholar] [CrossRef]
- Felitti, V.J.; And, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Koss, M.P.; Marks, J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef]
- Barnes, A.J.; Anthony, B.J.; Karatekin, C.; Lingras, K.A.; Mercado, R.; Thompson, L.A. Identifying adverse childhood experiences in pediatrics to prevent chronic health conditions. Pediatr. Res. 2020, 87, 362–370. [Google Scholar] [CrossRef]
- Lin, L.; Wang, H.H.; Lu, C.; Chen, W.; Guo, V.Y. Adverse Childhood Experiences and Subsequent Chronic Diseases Among Middle-aged or Older Adults in China and Associations With Demographic and Socioeconomic Characteristics. JAMA Netw. Open 2021, 4, e2130143. [Google Scholar] [CrossRef]
- Afifi, T.O.; Taillieu, T.; Salmon, S.; Davila, I.G.; Stewart-Tufescu, A.; Fortier, J.; Struck, S.; Asmundson, G.J.G.; Sareen, J.; MacMillan, H.L. Adverse childhood experiences (ACEs), peer victimization, and substance use among adolescents. Child Abuse Negl. 2020, 106, 104504. [Google Scholar] [CrossRef]
- Poole, J.C.; Kim, H.S.; Dobson, K.S.; Hodgins, D.C. Adverse Childhood Experiences and Disordered Gambling: Assessing the Mediating Role of Emotion Dysregulation. J. Gambl. Stud. 2017, 33, 1187–1200. [Google Scholar] [CrossRef]
- Pape, K.; Cowell, W.; Sejbaek, C.S.; Andersson, N.W.; Svanes, C.; Kolstad, H.A.; Liu, X.; Hougaard, K.S.; Wright, R.J.; Schlünssen, V. Adverse childhood experiences and asthma: Trajectories in a national cohort. Thorax 2021, 76, 547–553. [Google Scholar] [CrossRef]
- Ittoop, T.; Jeffrey, K.; Cheng, C.I.; Reddy, S. The Relationship Between Adverse Childhood Experiences and Diabetes in Central Michigan Adults. Endocr. Pract. 2020, 26, 1425–1434. [Google Scholar] [CrossRef]
- Godoy, L.C.; Frankfurter, C.; Cooper, M.; Lay, C.; Maunder, R.; Farkouh, M.E. Association of Adverse Childhood Experiences With Cardiovascular Disease Later in Life: A Review. JAMA Cardiol. 2021, 6, 228–235. [Google Scholar] [CrossRef]
- Dennison, M.J.; Rosen, M.L.; Sambrook, K.A.; Jenness, J.L.; Sheridan, M.A.; McLaughlin, K.A. Differential Associations of Distinct Forms of Childhood Adversity With Neurobehavioral Measures of Reward Processing: A Developmental Pathway to Depression. Child Dev. 2019, 90, e96–e113. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Kaminga, A.C.; Yang, J.; Liu, J.; Xu, H. Adverse childhood experiences and risk of cancer during adulthood: A systematic review and meta-analysis. Child Abuse Negl. 2021, 117, 105088. [Google Scholar] [CrossRef]
- Letourneau, N.; Dewey, D.; Kaplan, B.J.; Ntanda, H.; Novick, J.; Thomas, J.C.; Deane, A.J.; Leung, B.; Pon, K.; Giesbrecht, G.F.; et al. Intergenerational transmission of adverse childhood experiences via maternal depression and anxiety and moderation by child sex. J. Dev. Orig. Health Dis. 2019, 10, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.R.; Kwack, Y.S.; Song, J.K.; Kim, M.D.; Park, J.H.; Kim, B.N.; Moon, D.S. The Impact of Maternal Adverse Childhood Experiences on Offspring’s Internalizing and Externalizing Problems. Psychiatry Investig. 2021, 18, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Injury Prevention and Control: Division of Violence Prevention. Centers for Disease Control and Prevention. The ACE Pyramid. Available online: http://www.cdc.gov/violenceprevention/acestudy/pyramid.html (accessed on 13 November 2021).
- Dietert, R.R. Microbiome First Approaches to Rescue Public Health and Reduce Human Suffering. Biomedicines 2021, 9, 1581. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Preventing Adverse Childhood Experiences. Available online: https://www.cdc.gov/violenceprevention/aces/fastfact.html (accessed on 22 November 2021).
- Hughes, K.; Ford, K.; Bellis, M.A.; Glendinning, F.; Harrison, E.; Passmore, J. Health and financial costs of adverse childhood experiences in 28 European countries: A systematic review and meta-analysis. Lancet Public Health 2021, 6, e848–e857. [Google Scholar] [CrossRef]
- John-Henderson, N.A.; Henderson-Matthews, B.; Ollinger, S.R.; Racine, J.; Gordon, M.R.; Higgins, A.A.; Horn, W.C.; Reevis, S.A.; Running Wolf, J.A.; Grant, D.; et al. Adverse Childhood Experiences and Immune System Inflammation in Adults Residing on the Blackfeet Reservation: The Moderating Role of Sense of Belonging to the Community. Ann. Behav. Med. 2020, 54, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, C.A.; Johnson, R.L.; Freeman, E.W.; Sammel, M.D.; Epperson, C.N. Influences of the menopause transition and adverse childhood experiences on peripheral basal inflammatory markers. Brain Behav. Immun. Health 2021, 15, 100280. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.B.; Patil, N.D.; Meriaux, S.; Theresine, M.; Muller, C.P.; Leenen, F.A.D.; Elwenspoek, M.M.C.; Zimmer, J.; Turner, J.D. Unbiased Screening Identifies Functional Differences in NK Cells After Early Life Psychosocial Stress. Front. Immunol. 2021, 12, 674532. [Google Scholar] [CrossRef]
- Sanderson, M.; Mouton, C.P.; Cook, M.; Liu, J.; Blot, W.J.; Hargreaves, M.K. Adverse Childhood Experiences and Chronic Disease Risk in the Southern Community Cohort Study. J. Health Care Poor Underserved 2021, 32, 1384–1402. [Google Scholar] [CrossRef] [PubMed]
- Merrick, M.T.; Ford, D.C.; Ports, K.A.; Guinn, A.S.; Chen, J.; Klevens, J.; Metzler, M.; Jones, C.M.; Simon, T.R.; Daniel, V.M.; et al. Vital Signs: Estimated Proportion of Adult Health Problems Attributable to Adverse Childhood Experiences and Implications for Prevention-25 States, 2015-2017. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 999–1005. [Google Scholar] [CrossRef] [Green Version]
- Tang, R.; Howe, L.D.; Suderman, M.; Relton, C.L.; Crawford, A.A.; Houtepen, L.C. Adverse childhood experiences, DNA methylation age acceleration, and cortisol in UK children: A prospective population-based cohort study. Clin. Epigenet. 2020, 12, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merz, M.P.; Turner, J.D. Is early life adversity a trigger towards inflammageing? Exp. Gerontol. 2021, 150, 111377. [Google Scholar] [CrossRef]
- Rojo-Wissar, D.M.; Sosnowski, D.W.; Ingram, M.M.; Jackson, C.L.; Maher, B.S.; Alfano, C.A.; Meltzer, L.J.; Spira, A.P. Associations of adverse childhood experiences with adolescent total sleep time, social jetlag, and insomnia symptoms. Sleep Med. 2021, 88, 104–115. [Google Scholar] [CrossRef]
- Sullivan, K.; Rochani, H.; Huang, L.T.; Donley, D.K.; Zhang, J. Adverse childhood experiences affect sleep duration for up to 50 years later. Sleep 2019, 42, 87. [Google Scholar] [CrossRef]
- Park, E.J.; Kim, S.Y.; Kim, Y.; Sung, D.; Kim, B.; Hyun, Y.; Jung, K.I.; Lee, S.Y.; Kim, H.; Park, S.; et al. The Relationship between Adverse Childhood Experiences and Sleep Problems among Adolescent Students: Mediation by Depression or Anxiety. Int. J. Environ. Res. Public Health 2020, 18, 236. [Google Scholar] [CrossRef]
- April-Sanders, A.; Duarte, C.S.; Wang, S.; McGlinchey, E.; Alcántara, C.; Bird, H.; Canino, G.; Suglia, S.F. Childhood Adversity and Sleep Disturbances: Longitudinal Results in Puerto Rican Children. Int. J. Behav. Med. 2021, 28, 107–115. [Google Scholar] [CrossRef]
- Zilioli, S.; Slatcher, R.B.; Chi, P.; Li, X.; Zhao, J.; Zhao, G. Childhood Adversity, Self-Esteem, and Diurnal Cortisol Profiles Across the Life Span. Environ. Sci. 2016, 27, 1249–1265. [Google Scholar] [CrossRef] [Green Version]
- Bronsard, G.; Auquier, P.; Boyer, L. Links between early child maltreatment, mental disorders, and cortisol secretion anomalies. J. Physiol. Paris 2016, 110, 448–452. [Google Scholar] [CrossRef]
- Grillault Laroche, D.; Curis, E.; Bellivier, F.; Nepost, C.; Gross, G.; Etain, B.; Marie-Claire, C. Network of co-expressed circadian genes, childhood maltreatment and sleep quality in bipolar disorders. Chronobiol. Int. 2021, 38, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.P.; Tucker, J.S.; Stein, B.D.; D’Amico, E.J. Longitudinal effects of adverse childhood experiences on substance use transition patterns during young adulthood. Child. Abuse Negl. 2021, 120, 105201. [Google Scholar] [CrossRef]
- Rogers, C.J.; Forster, M.; Grigsby, T.J.; Albers, L.; Morales, C.; Unger, J.B. The impact of childhood trauma on substance use trajectories from adolescence to adulthood: Findings from a longitudinal Hispanic cohort study. Child Abuse Negl. 2021, 120, 105200. [Google Scholar] [CrossRef] [PubMed]
- Schafer, E.S. Adverse childhood experiences and risky behaviors in male college students. J. Am. Coll. Health 2021, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bussières, A.; Hartvigsen, J.; Ferreira, M.L.; Ferreira, P.H.; Hancock, M.J.; Stone, L.S.; Wideman, T.H.; Boruff, J.; Elklit, A. Adverse childhood experience and adult persistent pain and disability: Protocol for a systematic review and meta-analysis. Syst. Rev. 2020, 9, 215. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Xu, L.P.; Zeng, C.C.; Zhang, X.Y.; Tao, F.B.; Sun, Y. Prolonged parent-child separation and pain in adolescence: The role of HPA-axis genetic variations. J. Affect. Disord. 2021, 292, 255–260. [Google Scholar] [CrossRef]
- Salonsalmi, A.; Pietiläinen, O.; Lahelma, E.; Rahkonen, O.; Lallukka, T. Contributions of childhood adversities to chronic pain among mid-life employees. Scand. J. Public Health 2021, 18, 1403494820981509. [Google Scholar] [CrossRef]
- Walton, D.M.; Tremblay, P.; Seo, W.; Elliott, J.M.; Ghodrati, M.; May, C.; MacDermid, J.C. Effects of childhood trauma on pain-related distress in adults. Eur. J. Pain 2021, 25, 2166–2176. [Google Scholar] [CrossRef]
- Lopez, M.; Ruiz, M.O.; Rovnaghi, C.R.; Tam, G.K.; Hiscox, J.; Gotlib, I.H.; Barr, D.A.; Carrion, V.G.; Anand, K.J.S. The social ecology of childhood and early life adversity. Pediatr. Res. 2021, 89, 353–367. [Google Scholar] [CrossRef]
- Leeners, B.; Rath, W.; Block, E.; Görres, G.; Tschudin, S. Risk factors for unfavorable pregnancy outcome in women with adverse childhood experiences. J. Perinat. Med. 2014, 42, 171–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarei, K.; Xu, G.; Zimmerman, B.; Giannotti, M.; Strathearn, L. Adverse Childhood Experiences Predict Common Neurodevelopmental and Behavioral Health Conditions among U.S. Children. Children 2021, 8, 761. [Google Scholar] [CrossRef] [PubMed]
- Gehred, M.Z.; Knodt, A.R.; Ambler, A.; Bourassa, K.J.; Danese, A.; Elliott, M.L.; Hogan, S.; Ireland, D.; Poulton, R.; Ramrakha, S.; et al. Long-term Neural Embedding of Childhood Adversity in a Population-Representative Birth Cohort Followed for 5 Decades. Biol. Psychiatry 2021, 90, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.; Stubbings, D.R.; De Claire, K. Factors Predictive of Behavioural and Emotional Dysfunction in Adolescents in a Secure Children’s Home. J. Child. Adolesc Trauma 2020, 14, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, M.; Bertsch, K.; Löffler, A.; Steinmann, S.; Herpertz, S.C.; Bekrater-Bodmann, R. Body connection mediates the relationship between traumatic childhood experiences and impaired emotion regulation in borderline personality disorder. Bord. Pers. Disord. Emot. Dysregul. 2021, 8, 17. [Google Scholar] [CrossRef]
- Sachs-Ericsson, N.J.; Rushing, N.C.; Stanley, I.H.; Sheffler, J. In my end is my beginning: Developmental trajectories of adverse childhood experiences to late-life suicide. Aging Ment. Health 2016, 20, 139–165. [Google Scholar] [CrossRef]
- Sahle, B.W.; Reavley, N.J.; Li, W.; Morgan, A.J.; Yap, M.B.H.; Reupert, A.; Jorm, A.F. The association between adverse childhood experiences and common mental disorders and suicidality: An umbrella review of systematic reviews and meta-analyses. Eur. Child Adolesc. Psychiatry 2021. [Google Scholar] [CrossRef]
- Dempster, K.S.; O’Leary, D.D.; MacNeil, A.J.; Hodges, G.J.; Wade, T.J. Linking the hemodynamic consequences of adverse childhood experiences to an altered HPA axis and acute stress response. Brain Behav. Immun. 2021, 93, 254–263. [Google Scholar] [CrossRef]
- Marques-Feixa, L.; Palma-Gudiel, H.; Romero, S.; Moya-Higueras, J.; Rapado-Castro, M.; Castro-Quintas, Á.; Zorrilla, I.; José Muñoz, M.; Ramírez, M.; Mayoral, M.; et al. Childhood maltreatment disrupts HPA-axis activity under basal and stress conditions in a dose-response relationship in children and adolescents. Psychol. Med. 2021, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Organization International Classification of Diseases 11th. 2019. Available online: https://www.who.int/standards/classifications/classification-of-diseases (accessed on 22 November 2021).
- Guo, T.; Huang, L.; Hall, D.L.; Jiao, C.; Chen, S.T.; Yu, Q.; Yeung, A.; Chi, X.; Zou, L. The relationship between childhood adversities and complex posttraumatic stress symptoms: A multiple mediation model. Eur. J. Psychotraumatol. 2021, 12, 1936921. [Google Scholar] [CrossRef]
- Anastas, J.W.; Payne, N.A.; Ghuman, S.A. Adverse Childhood Experiences and Complex Post-traumatic Stress in Pregnant Teens: A Pilot Study. Matern. Child Health J. 2021, 25, 741–750. [Google Scholar] [CrossRef]
- Tian, Y.; Li, W.; Wu, X.; Cheng, X. Complex PTSD in Chinese Adolescents Exposed to Childhood Trauma: A Latent Profile Analysis. J. Interpers. Violence 2021, 8862605211050111. [Google Scholar] [CrossRef] [PubMed]
- Hantsoo, L.; Jašarević, E.; Criniti, S.; McGeehan, B.; Tanes, C.; Sammel, M.D.; Elovitz, M.A.; Compher, C.; Wu, G.; Epperson, C.N. Childhood adversity impact on gut microbiota and inflammatory response to stress during pregnancy. Brain Behav. Immun. 2019, 75, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Hantsoo, L.; Zemel, B.S. Stress gets into the belly: Early life stress and the gut microbiome. Behav. Brain Res. 2021, 414, 113474. [Google Scholar] [CrossRef] [PubMed]
- Dietert, R.R. The microbiome-immune-host defense barrier complex (microimmunosome) and developmental programming of noncommunicable diseases. Reprod. Toxicol. 2017, 68, 49–58. [Google Scholar] [CrossRef]
- Dietert, R.R. Microbiome First Medicine in Health and Safety. Biomedicines 2021, 9, 1099. [Google Scholar] [CrossRef]
- World Health Organization. Noncommunicable Diseases. Version 13 April 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 5 December 2021).
- Rahal, H.; Videlock, E.J.; Icenhour, A.; Shih, W.; Naliboff, B.; Gupta, A.; Mayer, E.A.; Chang, L. Importance of trauma-related fear in patients with irritable bowel syndrome and early adverse life events. Neurogastroenterol. Motil. 2020, 32, e13896. [Google Scholar] [CrossRef]
- Wing, R.; Gjelsvik, A.; Nocera, M.; McQuaid, E.L. Association between adverse childhood experiences in the home and pediatric asthma. Ann. Allergy Asthma Immunol. 2015, 114, 379–384. [Google Scholar] [CrossRef]
- Ospina, M.B.; Serrano-Lomelin, J.A.; Amjad, S.; Hicks, A.; Giesbrecht, G.F. Latent factors of adverse childhood experiences and adult-onset asthma. J. Dev. Orig. Health Dis. 2021, 12, 50–57. [Google Scholar] [CrossRef]
- Brew, B.K.; Lundholm, C.; Osvald, E.C.; Chambers, G.; Öberg, A.S.; Fang, F.; Almqvis, T.C. Early life adversity due to bereavement and inflammatory diseases in the next generation–A population study in transgenerational stress exposure. Am. J. Epidemiol. 2021, 236. [Google Scholar] [CrossRef]
- Jimenez, V.; Sanchez, N.; Clark, E.L.M.; Miller, R.L.; Casamassima, M.; Verros, M.; Conte, I.; Ruiz-Jaquez, M.; Gulley, L.D.; Johnson, S.A.; et al. Associations of adverse childhood experiences with stress physiology and insulin resistance in adolescents at risk for adult obesity. Dev. Psychobiol. 2021, 63, e22127. [Google Scholar] [CrossRef]
- Rafiq, T.; O’Leary, D.D.; Dempster, K.S.; Cairney, J.; Wade, T.J. Adverse Childhood Experiences (ACEs) Predict Increased Arterial Stiffness from Childhood to Early Adulthood: Pilot Analysis of the Niagara Longitudinal Heart Study. J. Child Adolesc. Trauma 2020, 13, 505–514. [Google Scholar] [CrossRef]
- Akamine, A.A.; Rusch, G.S.; Nisihara, R.; Skare, T.L. Adverse childhood experience in patients with psoriasis. Trends Psychiatry Psychother. 2021. [Google Scholar] [CrossRef]
- Deschênes, S.S.; Kivimaki, M.; Schmitz, N. Adverse Childhood Experiences and the Risk of Coronary Heart Disease in Adulthood: Examining Potential Psychological, Biological, and Behavioral Mediators in the Whitehall II Cohort Study. J. Am. Heart Assoc. 2021, 10, e019013. [Google Scholar] [CrossRef]
- Saya, A.; Proietti, L.; Lisi, G.; Ribolsi, M.; Uccioli, L.; Niolu, C.; Siracusano, A. Traumatic experiences and type 2 diabetes mellitus. Riv. Psichiatr. 2020, 55, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Dube, S.R.; Fairweather, D.; Pearson, W.S.; Felitti, V.J.; Anda, R.F.; Croft, J.B. Cumulative childhood stress and autoimmune diseases in adults. Psychosom. Med. 2009, 71, 243–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cozier, Y.C.; Barbhaiya, M.; Castro-Webb, N.; Conte, C.; Tedeschi, S.; Leatherwood, C.; Costenbader, K.H.; Rosenberg, L. Association of Child Abuse and Systemic Lupus Erythematosus in Black Women During Adulthood. Arthritis Care Res. 2021, 73, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Feldman, C.H.; Malspeis, S.; Leatherwood, C.; Kubzansky, L.; Costenbader, K.H.; Roberts, A.L. Association of Childhood Abuse with Incident Systemic Lupus Erythematosus in Adulthood in a Longitudinal Cohort of Women. J. Rheumatol. 2019, 46, 1589–1596. [Google Scholar] [CrossRef]
- Gielen, S.; Janmohamed, S.R.; Van Laethem, A.; Del Marmol, V.; Suppa, M.; Gutermuth, J.; Willemsen, R. Hidradenitis suppurativa is associated with childhood and lifetime traumatic events: A case-control study. J. Eur. Acad Dermatol. Venereol. 2020, 34, 2877–2883. [Google Scholar] [CrossRef]
- Kelly-Irving, M.; Lepage, B.; Dedieu, D.; Lacey, R.; Cable, N.; Bartley, M.; Blane, D.; Grosclaude, P.; Lang, T.; Delpierre, C. Childhood adversity as a risk for cancer: Findings from the 1958 British birth cohort study. BMC Public Health 2013, 13, 767. [Google Scholar] [CrossRef]
- Ayaydin, H.; Abali, O.; Akdeniz, N.O.; Kok, B.E.; Gunes, A.; Yildirim, A.; Deniz, G. Immune system changes after sexual abuse in adolescents. Pediatr. Int. 2016, 58, 105–112. [Google Scholar] [CrossRef]
- Kuzminskaite, E.; Vinkers, C.H.; Elzinga, B.M.; Wardenaar, K.; Giltay, E.J.; Penninx, B.W.J.H. Childhood trauma and dysregulation of multiple biological stress systems in adulthood: Results from the Netherlands Study of Depression and Anxiety (NESDA). Psychoneuroendocrinology 2020, 121, 104835. [Google Scholar] [CrossRef]
- Trotta, A.; Arseneault, L.; Danese, A.; Mondelli, V.; Rasmussen, L.J.H.; Fisher, H.L. Associations between childhood victimization, inflammatory biomarkers and psychotic phenomena in adolescence: A longitudinal cohort study. Brain Behav. Immun. 2021, 98, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fan, Q.; Nicholas, S.; Maitland, E. The long arm of childhood: The prolonged influence of adverse childhood experiences on depression during middle and old age in China. J. Health Psychol. 2021, 16, 13591053211037727. [Google Scholar] [CrossRef]
- Quidé, Y.; Tozzi, L.; Corcoran, M.; Cannon, D.M.; Dauvermann, M.R. The Impact of Childhood Trauma on Developing Bipolar Disorder: Current Understanding and Ensuring Continued Progress. Neuropsychiatr. Dis. Treat. 2020, 16, 3095–3115. [Google Scholar] [CrossRef] [PubMed]
- Schickedanz, H.B.; Jennings, L.A.; Schickedanz, A. The Association Between Adverse Childhood Experiences and Positive Dementia Screen in American Older Adults. J. Gen. Intern. Med. 2021. [Google Scholar] [CrossRef]
- Liebermann, C.; Kohl Schwartz, A.S.; Charpidou, T.; Geraedts, K.; Rauchfuss, M.; Wölfler, M.; von Orelli, S.; Häberlin, F.; Eberhard, M.; Imesch, P.; et al. Maltreatment during childhood: A risk factor for the development of endometriosis? Hum. Reprod. 2018, 33, 1449–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, G.; Oh, J. The Relationship between Childhood Trauma, Eating Disorders, and Sleep Quality among Registered Hospital Nurses in South Korea. Healthcare 2020, 8, 490. [Google Scholar] [CrossRef] [PubMed]
- Schmahl, O.C.; Jeuring, H.W.; Aprahamian, I.; Naarding, P.; Marijnissen, R.M.; Hendriks, G.J.; Fluiter, M.; Rhebergen, D.; Lugtenburg, A.; Lammers, M.W.; et al. Impact of childhood trauma on multidimensional frailty in older patients with a unipolar depressive-, anxiety- or somatic symptom disorder. Arch. Gerontol. Geriatr. 2021, 96, 104452. [Google Scholar] [CrossRef] [PubMed]
- Kratzer, L.; Knefel, M.; Haselgruber, A.; Heinz, P.; Schennach, R.; Karatzias, T. Co-occurrence of severe PTSD, somatic symptoms and dissociation in a large sample of childhood trauma inpatients: A network analysis. Eur. Arch. Psychiatry Clin. Neurosci. 2021. [Google Scholar] [CrossRef]
- Hossack, M.R.; Reid, M.W.; Aden, J.K.; Gibbons, T.; Noe, J.C.; Willis, A.M. Adverse Childhood Experience, Genes, and PTSD Risk in Soldiers: A Methylation Study. Mil. Med. 2020, 185, 377–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boterhoven de Haan, K.L.; Lee, C.W.; Fassbinder, E.; van Es, S.M.; Menninga, S.; Meewisse, M.L.; Rijkeboer, M.; Kousemaker, M.; Arntz, A. Imagery rescripting and eye movement desensitisation and reprocessing as treatment for adults with post-traumatic stress disorder from childhood trauma: Randomised clinical trial. Br. J. Psychiatry 2020, 217, 609–615. [Google Scholar] [CrossRef]
- Kliewer, W.; Robins, J.L. Adverse Childhood Experiences Are Associated with Cardiometabolic Risk Indicators and Telomere Length in Low-Income African-American Adolescents. Int. J. Behav. Med. 2021. [Google Scholar] [CrossRef]
- Xavier, G.; Spindola, L.M.; Ota, V.K.; Carvalho, C.M.; Maurya, P.K.; Tempaku, P.F.; Moretti, P.N.; Mazotti, D.R.; Sato, J.R.; Brietzke, E.; et al. Effect of male-specific childhood trauma on telomere length. J. Psychiatr. Res. 2018, 107, 104–109. [Google Scholar] [CrossRef]
- Huffhines, L.; Jackson, Y.; McGuire, A.; Schreier, H.M.C. The intergenerational interplay of adversity on salivary inflammation in young children and caregivers. Psychoneuroendocrinology 2021, 128, 105222. [Google Scholar] [CrossRef]
- Lacey, R.E.; Pinto Pereira, S.M.; Li, L.; Danese, A. Adverse childhood experiences and adult inflammation: Single adversity, cumulative risk and latent class approaches. Brain Behav. Immun. 2020, 87, 820–830. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, A.; He, H.; Zhao, X.; Tao, F.; Sun, Y. Inflammatory burden in adolescents with prolonged parent-child separation. Brain Behav. Immun. 2021, 98, 257–262. [Google Scholar] [CrossRef]
- Rincel, M.; Aubert, P.; Chevalier, J.; Grohard, P.A.; Basso, L.; Monchaux de Oliveira, C.; Helbling, J.C.; Lévy, É.; Chevalier, G.; Leboyer, M.; et al. Multi-hit early life adversity affects gut microbiota, brain and behavior in a sex-dependent manner. Brain Behav. Immun. 2019, 80, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Dietert, R.R.; Dietert, J.M. Microbiome First Approaches in Pain Prevention and Management. Am. J. Biomed. Sci. Res. 2021, 14, 184–192. [Google Scholar] [CrossRef]
- Groenewald, C.B.; Murray, C.B.; Palermo, T.M. Adverse childhood experiences and chronic pain among children and adolescents in the United States. Pain Rep. 2020, 5, e839. [Google Scholar] [CrossRef] [PubMed]
- Dworsky-Fried, Z.; Kerr, B.J.; Taylor, A.M.W. Microbes, microglia, and pain. Neurobiol. Pain 2020, 7, 100045. [Google Scholar] [CrossRef]
- Santoni, M.; Miccini, F.; Battelli, N. Gut microbiota, immunity and pain. Immunol. Lett. 2021, 229, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Crock, L.W.; Baldridge, M.T. A role for the microbiota in complex regional pain syndrome? Neurobiol. Pain 2020, 8, 100054. [Google Scholar] [CrossRef] [PubMed]
- Arzani, M.; Jahromi, S.R.; Ghorbani, Z.; Vahabizad, F.; Martelletti, P.; Ghaemi, A.; Sacco, S.; Togha, M. School of Advanced Studies of the European Headache Federation (EHF-SAS). Gut-brain Axis and migraine headache: A comprehensive review. J. Headache Pain 2020, 21, 15. [Google Scholar] [CrossRef] [Green Version]
- Minerbi, A.; Fitzcharles, M.A. Gut microbiome: Pertinence in fibromyalgia. Clin. Exp. Rheumatol. 2020, 38, 99–104. [Google Scholar]
- van Thiel, I.A.M.; Botschuijver, S.; de Jonge, W.J.; Seppen, J. Painful interactions: Microbial compounds and visceral pain. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165534. [Google Scholar] [CrossRef]
- Pusceddu, M.M.; Gareau, M.G. Visceral pain: Gut microbiota, a new hope? J. Biomed. Sci. 2018, 25, 73. [Google Scholar] [CrossRef]
- Jadrešin, O.; Hojsak, I.; Mišak, Z.; Kekez, A.J.; Trbojević, T.; Ivković, L.; Kolaček, S. Lactobacillus reuteri DSM 17938 in the Treatment of Functional Abdominal Pain in Children: RCT Study. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 925–929. [Google Scholar] [CrossRef]
- Jadrešin, O.; Sila, S.; Trivić, I.; Mišak, Z.; Kolaček, S.; Hojsak, I. Lactobacillus reuteri DSM 17938 is effective in the treatment of functional abdominal pain in children: Results of the double-blind randomized study. Clin. Nutr. 2020, 39, 3645–3651. [Google Scholar] [CrossRef] [PubMed]
- Trivić, I.; Niseteo, T.; Jadrešin, O.; Hojsak, I. Use of probiotics in the treatment of functional abdominal pain in children-systematic review and meta-analysis. Eur. J. Pediatr. 2021, 180, 339–351. [Google Scholar] [CrossRef]
- Morelli, N.M.; Liuzzi, M.T.; Duong, J.B.; Kryza-Lacombe, M.; Chad-Friedman, E.; Villodas, M.T.; Dougherty, L.R.; Wiggins, J.L. Reward-related neural correlates of early life stress in school-aged children. Dev. Cogn. Neurosci. 2021, 49, 100963. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yu, Q.; Owen, C.E.; Ibarra Aspe, G.; Wiggins, J.L. Contributions of childhood abuse and neglect to reward neural substrates in adolescence. Neuroimage Clin. 2021, 32, 102832. [Google Scholar] [CrossRef] [PubMed]
- Levitt, E.E.; Amlung, M.T.; Gonzalez, A.; Oshri, A.; MacKillop, J. Consistent evidence of indirect effects of impulsive delay discounting and negative urgency between childhood adversity and adult substance use in two samples. Psychopharmacology 2021, 238, 2011–2020. [Google Scholar] [CrossRef]
- Georgsdottir, M.T.; Sigurdardottir, S.; Gunnthorsdottir, H. “This Is the Result of Something Else”: Experiences of Men That Abused Drugs and Had Experienced Childhood Trauma. Am. J. Mens. Health 2021, 15, 15579883211009348. [Google Scholar] [CrossRef]
- Fernandes, G.S.; Spiers, A.; Vaidya, N.; Zhang, Y.; Sharma, E.; Holla, B.; Heron, J.; Hickman, M.; Murthy, P.; Chakrabarti, A.; et al. Adverse childhood experiences and substance misuse in young people in India: Results from the multisite cVEDA cohort. BMC Public Health 2021, 21, 1920. [Google Scholar] [CrossRef]
- Villamil Grest, C.; Cederbaum, J.A.; Lee, J.O.; Unger, J.B. Adverse childhood experiences and the substance use behaviors of Latinx youth. Drug Alcohol Depend. 2021, 227, 108936. [Google Scholar] [CrossRef]
- Giano, Z.; O’Neil, A.; Hubach, R.D. The Effects of Individual and Dual ACE Domains on Binge Drinking: Comparisons by Gender. Subst. Use Misuse 2021, 56, 1642–1650. [Google Scholar] [CrossRef]
- Pakdaman, S.; Unger, J.B.; Forster, M.; Rogers, C.J.; Sussman, S.Y.; Benjamin, S.M. Childhood Trauma and Prescription Drug Misuse in a College Population. Subst. Use Misuse 2021, 56, 140–144. [Google Scholar] [CrossRef]
- Dietert, R.R.; DeWitt, J.C.; Germolec, D.R.; Zelikoff, J.T. Breaking patterns of environmentally influenced disease for health risk reduction: Immune perspectives. Environ. Health Persp. 2010, 118, 1091–1099. [Google Scholar] [CrossRef]
- Atger, F.; Mauvoisin, D.; Weger, B.; Gobet, C.; Gachon, F. Regulation of Mammalian Physiology by Interconnected Circadian and Feeding Rhythms. Front. Endocrinol. 2017, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- de Assis, L.V.M.; Oster, H. The circadian clock and metabolic homeostasis: Entangled networks. Cell Mol. Life Sci. 2021, 78, 4563–4587. [Google Scholar] [CrossRef] [PubMed]
- Boyce, W.T.; Levitt, P.; Martinez, F.M.; McEwen, B.S.; Shonkoff, J.P. Genes, Environments, and Time: The Biology of Adversity and Resilience. Pediatrics 2021, 147, e20201651. [Google Scholar] [CrossRef]
- Preußner, M.; Heyd, F. Post-transcriptional control of the mammalian circadian clock: Implications for health and disease. Pflugers Arch. 2016, 468, 983–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.D.; Xin, H.; Yuan, Y.; Yang, X.; Li, H.; Tian, D.; Zhang, H.; Zhang, Z.; Han, T.L.; Chen, Q.; et al. Circadian Clock-Controlled Checkpoints in the Pathogenesis of Complex Disease. Front. Genet. 2021, 12, 721231. [Google Scholar] [CrossRef]
- Hemmer, A.; Mareschal, J.; Dibner, C.; Pralong, J.A.; Dorribo, V.; Perrig, S.; Genton, L.; Pichard, C.; Collet, T.H. The Effects of Shift Work on Cardio-Metabolic Diseases and Eating Patterns. Nutrients 2021, 13, 4178. [Google Scholar] [CrossRef]
- Duan, C.; Jenkins, Z.M.; Castle, D. Therapeutic use of melatonin in schizophrenia: A systematic review. World J. Psychiatry 2021, 11, 463–476. [Google Scholar] [CrossRef]
- Gulick, D.; Gamsby, J.J. Racing the clock: The role of circadian rhythmicity in addiction across the lifespan. Pharmacol Ther. 2018, 188, 124–139. [Google Scholar] [CrossRef]
- Scott, M.R.; McClung, C.A. Circadian Rhythms in Mood Disorders. Adv. Exp. Med. Biol. 2021, 1344, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Sassone-Corsi, P. Linking Depression to Epigenetics: Role of the Circadian Clock. Adv. Exp. Med. Biol. 2021, 1344, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Bumgarner, J.R.; Walker, W.H., II; Nelson, R.J. Circadian rhythms and pain. Neurosci. Biobehav. Rev. 2021, 129, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Li, L. Circadian Clock Regulates Inflammation and the Development of Neurodegeneration. Front. Cell. Infect. Microbiol. 2021, 11, 696554. [Google Scholar] [CrossRef]
- Kawai, M. Disruption of the circadian rhythms and its relationship with pediatric obesity. Pediatr. Int. 2021. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Mota, S.; Zhang, B. Circadian Clock Regulation on Lipid Metabolism and Metabolic Diseases. Adv. Exp. Med. Biol. 2020, 1276, 53–66. [Google Scholar] [CrossRef]
- Cable, J.; Schernhammer, E.; Hanlon, E.C.; Vetter, C.; Cedernaes, J.; Makarem, N.; Dashti, H.S.; Shechter, A.; Depner, C.; Ingiosi, A.; et al. Sleep and circadian rhythms: Pillars of health-a Keystone Symposia report. Ann. N. Y. Acad. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.L.; Miller, L.; Yi, H.; Keil, R.; Handa, R.J.; Wu, T.J. Knockout of the circadian gene, Per2, disrupts corticosterone secretion and results in depressive-like behaviors and deficits in startle responses. BMC Neurosci. 2021, 22, 5. [Google Scholar] [CrossRef]
- Peñalvo, J.L.; Mertens, E.; Muñoz-Cabrejas, A.; León-Latre, M.; Jarauta, E.; Laclaustra, M.; Ordovás, J.M.; Casasnovas, J.A.; Uzhova, I.; Moreno-Franco, B. Work Shift, Lifestyle Factors, and Subclinical Atherosclerosis in Spanish Male Workers: A Mediation Analysis. Nutrients 2021, 13, 1077. [Google Scholar] [CrossRef]
- Duan, J.; Greenberg, E.N.; Karri, S.S.; Andersen, B. The circadian clock and diseases of the skin. FEBS Lett. 2021, 595, 2413–2436. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, S.; Huang, X.; Chai, R.; Tang, Q.; Yang, R.; Huang, X.; Wang, X.; Zheng, K. Dysregulation of Circadian Clock Genes as Significant Clinic Factor in the Tumorigenesis of Hepatocellular Carcinoma. Comput. Math. Methods Med. 2021, 8238833. [Google Scholar] [CrossRef]
- Maidstone, R.J.; Turner, J.; Vetter, C.; Dashti, H.S.; Saxena, R.; Scheer, F.A.J.L.; Shea, S.A.; Kyle, S.D.; Lawlor, D.A.; Loudon, A.S.I.; et al. Night shift work is associated with an increased risk of asthma. Thorax 2021, 76, 53–60. [Google Scholar] [CrossRef]
- Nakao, A. Circadian Regulation of the Biology of Allergic Disease: Clock Disruption Can Promote Allergy. Front. Immunol. 2020, 11, 1237. [Google Scholar] [CrossRef]
- Agrawal, R.; Ajami, N.J.; Malhotra, S.; Chen, L.; White, D.L.; Sharafkhaneh, A.; Hoffman, K.L.; Graham, D.Y.; El-Serag, H.B.; Petrosino, J.F.; et al. Habitual Sleep Duration and the Colonic Mucosa-Associated Gut Microbiota in Humans—A Pilot Study. Clocks Sleep 2021, 3, 387–397. [Google Scholar] [CrossRef]
- Butler, T.D.; Gibbs, J.E. Circadian Host-Microbiome Interactions in Immunity. Front. Immunol. 2020, 11, 1783. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Rao, M.C.; Chang, E.B. Gut microbiota as a transducer of dietary cues to regulate host circadian rhythms and metabolism. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Costantini, C.; Renga, G.; Sellitto, F.; Borghi, M.; Stincardini, C.; Pariano, M.; Zelante, T.; Chiarotti, F.; Bartoli, A.; Mosci, P.; et al. Microbes in the Era of Circadian Medicine. Front. Cell. Infect. Microbiol. 2020, 10, 30. [Google Scholar] [CrossRef]
- Daas, M.C.; de Roos, N.M. Intermittent fasting contributes to aligned circadian rhythms through interactions with the gut microbiome. Benef. Microbes 2021, 12, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, S.L.; Engen, P.A.; Naqib, A.; Qian, J.; Vujovic, N.; Rahman, N.; Green, S.J.; Garaulet, M.; Keshavarzian, A.; Scheer, F.A.J.L. Proof-of-principle demonstration of endogenous circadian system and circadian misalignment effects on human oral microbiota. FASEB J. 2022, 36, e22043. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Kang, X.; Cai, Y. The gut microbiome as a target for adjuvant therapy in insomnia disorder. Clin. Res. Hepatol. Gastroenterol. 2021, 101834. [Google Scholar] [CrossRef]
- Ho, Y.T.; Tsai, Y.C.; Kuo, T.B.J.; Yang, C.C.H. Effects of Lactobacillus plantarum PS128 on Depressive Symptoms and Sleep Quality in Self-Reported Insomniacs: A Randomized, Double-Blind, Placebo-Controlled Pilot Trial. Nutrients 2021, 13, 2820. [Google Scholar] [CrossRef]
- Moloney, G.M.; Long-Smith, C.M.; Murphy, A.; Dorland, D.; Hojabri, S.F.; Ramirez, L.O.; Marin, D.C.; Bastiaanssen, T.F.S.; Cusack, A.M.; Berding, K.; et al. Improvements in sleep indices during exam stress due to consumption of a Bifidobacterium longum. Brain Behav. Immun. Health 2020, 10, 100174. [Google Scholar] [CrossRef]
- Lee, H.J.; Hong, J.K.; Kim, J.K.; Kim, D.H.; Jang, S.W.; Han, S.W.; Yoon, I.Y. Effects of Probiotic NVP-1704 on Mental Health and Sleep in Healthy Adults: An 8-Week Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 2660. [Google Scholar] [CrossRef] [PubMed]
- Fei, N.; Choo-Kang, C.; Reutrakul, S.; Crowley, S.J.; Rae, D.; Bedu-Addo, K.; Plange-Rhule, J.; Forrester, T.E.; Lambert, E.V.; Bovet, P.; et al. Gut microbiota alterations in response to sleep length among African-origin adults. PLoS ONE 2021, 16, e0255323. [Google Scholar] [CrossRef]
- Kwon, H.; Chae, S.H.; Jung, H.J.; Shin, H.M.; Ban, O.H.; Yang, J.; Kim, J.H.; Jeong, J.E.; Jeon, H.M.; Kang, Y.W.; et al. The effect of probiotics supplementation in postoperative cancer patients: A prospective pilot study. Ann. Surg. Treat. Res. 2021, 101, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Nobile, V.; Giardina, S.; Puoci, F. The Effect of a Probiotic Complex on the Gut-Brain Axis: A Translational Study. Neuropsychobiology 2021, 30, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; van de Wouw, M.; Drogos, L.; Vaghef-Mehrabani, E.; Reimer, R.A.; Tomfohr-Madsen, L.; Giesbrecht, G.F. Sleep and the gut microbiota in preschool aged children. Sleep 2022, 17, zsac020. [Google Scholar] [CrossRef]
- Okuyama, Y.; Okamoto, T.; Sasaki, D.; Ozaki, K.; Songee, J.; Hatakeyama, S.; Mikami, T.; Ohyama, C. The influence of gut microbiome on progression of overactive bladder symptoms: A community-based 3-year longitudinal study in Aomori, Japan. Int. Urol. Nephrol. 2021. [Google Scholar] [CrossRef]
- Li, Y.; Shao, L.; Mou, Y.; Zhang, Y.; Ping, Y. Sleep, circadian rhythm and gut microbiota: Alterations in Alzheimer’s disease and their potential links in the pathogenesis. Gut Microbes 2021, 13, 1957407. [Google Scholar] [CrossRef]
- Neroni, B.; Evangelisti, M.; Radocchia, G.; Di Nardo, G.; Pantanella, F.; Villa, M.P.; Schippa, S. Relationship between sleep disorders and gut dysbiosis: What affects what? Sleep Med. 2021, 87, 1–7. [Google Scholar] [CrossRef]
- Withrow, D.; Bowers, S.J.; Depner, C.M.; González, A.; Reynolds, A.C.; Wright, K.P., Jr. Sleep and Circadian Disruption and the Gut Microbiome-Possible Links to Dysregulated Metabolism. Curr. Opin. Endocr. Metab. Res. 2021, 17, 26–37. [Google Scholar] [CrossRef]
- Sgro, M.; Kodila, Z.N.; Brady, R.D.; Reichelt, A.C.; Mychaisuk, R.; Yamakawa, G.R. Synchronizing Our Clocks as We Age: The Influence of the Brain-Gut-Immune Axis on the Sleep-Wake Cycle Across the Lifespan. Sleep 2021. [Google Scholar] [CrossRef]
- Wagner, K.H.; Cameron-Smith, D.; Wessner, B.; Franzke, B. Biomarkers of Aging: From Function to Molecular Biology. Nutrients 2016, 8, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erusalimsky, J.D. Oxidative stress, telomeres and cellular senescence: What non-drug interventions might break the link? Free Radic. Biol. Med. 2020, 150, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Bazaz, M.R.; Balasubramanian, R.; Monroy-Jaramillo, N.; Dandekar, M.P. Linking the Triad of Telomere Length, Inflammation, and Gut Dysbiosis in the Manifestation of Depression. ACS Chem. Neurosci. 2021, 12, 3516–3526. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, Z.; Zhao, J.; Schank, M.; Cao, D.; Dang, X.; Nguyen, L.N.; Nguyen, L.N.T.; Khanal, S.; Zhang, J.; et al. Selective oxidative stress induces dual damage to telomeres and mitochondria in human T cells. Aging Cell 2021, 9, e13513. [Google Scholar] [CrossRef]
- Hecker, M.; Bühring, J.; Fitzner, B.; Rommer, P.S.; Zettl, U.K. Genetic, Environmental and Lifestyle Determinants of Accelerated Telomere Attrition as Contributors to Risk and Severity of Multiple Sclerosis. Biomolecules 2021, 11, 1510. [Google Scholar] [CrossRef]
- Jose, S.S.; Bendickova, K.; Kepak, T.; Krenova, Z.; Fric, J. Chronic Inflammation in Immune Aging: Role of Pattern Recognition Receptor Crosstalk with the Telomere Complex? Front. Immunol. 2017, 8, 1078. [Google Scholar] [CrossRef] [Green Version]
- Chakravarti, D.; Hu, B.; Mao, X.; Rashid, A.; Li, J.; Li, J.; Liao, W.T.; Whitley, E.M.; Dey, P.; Hou, P.; et al. Telomere dysfunction activates YAP1 to drive tissue inflammation. Nat. Commun. 2020, 11, 4766. [Google Scholar] [CrossRef]
- Manchia, M.; Paribello, P.; Arzedi, C.; Bocchetta, A.; Caria, P.; Cocco, C.; Congiu, D.; Cossu, E.; Dettori, T.; Frau, D.V.; et al. A multidisciplinary approach to mental illness: Do inflammation, telomere length and microbiota form a loop? A protocol for a cross-sectional study on the complex relationship between inflammation, telomere length, gut microbiota and psychiatric disorders. BMJ Open 2020, 10, e032513. [Google Scholar] [CrossRef] [Green Version]
- Cakala-Jakimowicz, M.; Kolodziej-Wojnar, P.; Puzianowska-Kuznicka, M. Aging-Related Cellular, Structural and Functional Changes in the Lymph Nodes: A Significant Component of Immunosenescence? An Overview. Cells 2021, 10, 3148. [Google Scholar] [CrossRef]
- Fahy, G.M.; Brooke, R.T.; Watson, J.P.; Good, Z.; Vasanawala, S.S.; Maecker, H.; Leipold, M.D.; Lin, D.T.S.; Kobor, M.S.; Horvath, S. Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell 2019, 18, e13028. [Google Scholar] [CrossRef] [Green Version]
- Garmany, A.; Yamada, S.; Terzic, A. Longevity leap: Mind the healthspan gap. NPJ Regen. Med. 2021, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Juncan, A.M.; Moisă, D.G.; Santini, A.; Morgovan, C.; Rus, L.L.; Vonica-Țincu, A.L.; Loghin, F. Advantages of Hyaluronic Acid and Its Combination with Other Bioactive Ingredients in Cosmeceuticals. Molecules 2021, 26, 4429. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Ai, X.; Fu, T.; Ren, L.; Shang, Q.; Li, G.; Yu, G. In vitro fermentation of hyaluronan by human gut microbiota: Changes in microbiota community and potential degradation mechanism. Carbohydr. Polym. 2021, 269, 118313. [Google Scholar] [CrossRef] [PubMed]
- Puviani, M.; Campione, E.; Offidani, A.M.; De Grandi, R.; Bianchi, L.; Bobyr, I.; Giannoni, M.; Campanati, A.; Bottagisio, M.; Bidossi, A.; et al. Effects of a cream containing 5% hyaluronic acid mixed with a bacterial-wall-derived glycoprotein, glycyrretinic acid, piroctone olamine and climbazole on signs, symptoms and skin bacterial microbiota in subjects with seborrheic dermatitis of the face. Clin. Cosmet. Investig. Dermatol. 2019, 12, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Lv, Y.; Sun, Y.; Wang, Y.; Zhao, Z.; Shi, C.; Chen, X.; Wang, L.; Zhang, M.; Wei, B.; et al. The anti-inflammatory activity of specific-sized hyaluronic acid oligosaccharides. Carbohydr. Polym. 2022, 276, 118699. [Google Scholar] [CrossRef]
- Kotla, N.G.; Isa, I.L.M.; Rasala, S.; Demir, S.; Singh, R.; Baby, B.V.; Swamy, S.K.; Dockery, P.; Jala, V.R.; Rochev, Y.; et al. Modulation of Gut Barrier Functions in Ulcerative Colitis by Hyaluronic Acid System. Adv. Sci. 2021, e2103189. [Google Scholar] [CrossRef]
- Lee, C.H.; Chiang, C.F.; Kuo, F.C.; Su, S.C.; Huang, C.L.; Liu, J.S.; Lu, C.H.; Hsieh, C.H.; Wang, C.C.; Lee, C.H.; et al. High-Molecular-Weight Hyaluronic Acid Inhibits IL-1β-Induced Synovial Inflammation and Macrophage Polarization through the GRP78-NF-κB Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 11917. [Google Scholar] [CrossRef]
- Peng, X.; Hao, M.; Zhao, Y.; Cai, Y.; Chen, X.; Chen, H.; Zhang, Y.; Dong, L.; Liu, X.; Ding, C.; et al. Red ginseng has stronger anti-aging effects compared to ginseng possibly due to its regulation of oxidative stress and the gut microbiota. Phytomedicine 2021, 93, 153772. [Google Scholar] [CrossRef]
- de Luna Freire, M.O.; do Nascimento, L.C.P.; de Oliveira, K.Á.R.; de Oliveira, A.M.; Dos Santos Lima, M.; Napoleão, T.H.; da Costa Silva, J.H.; Lagranha, C.J.; de Souza, E.L.; de Brito Alves, J.L. Limosilactobacillus fermentum Strains with Claimed Probiotic Properties Exert Anti-oxidant and Anti-inflammatory Properties and Prevent Cardiometabolic Disorder in Female Rats Fed a High-Fat Diet. Probiotics Antimicrob. Proteins 2021. [Google Scholar] [CrossRef]
- Zhou, F.; Li, Y.L.; Zhang, X.; Wang, K.B.; Huang, J.A.; Liu, Z.H.; Zhu, M.Z. Polyphenols from Fu Brick Tea Reduce Obesity via Modulation of Gut Microbiota and Gut Microbiota-Related Intestinal Oxidative Stress and Barrier Function. J. Agric. Food Chem. 2021. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. A Review on Anti-Aging Properties of Probiotics. Int. J. Appl. Pharmaceut. 2018, 10, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Ilesanmi-Oyelere, B.L.; Roy, N.C.; Kruger, M.C. Modulation of Bone and Joint Biomarkers, Gut Microbiota, and Inflammation Status by Synbiotic Supplementation and Weight-Bearing Exercise: Human Study Protocol for a Randomized Controlled Trial. JMIR Res. Protoc. 2021, 10, e30131. [Google Scholar] [CrossRef]
- Landete, J.M.; Gaya, P.; Rodríguez, E.; Langa, S.; Peirotén, Á.; Medina, M.; Arqués, J.L. Probiotic Bacteria for Healthier Aging: Immunomodulation and Metabolism of Phytoestrogens. Biomed. Res. Int. 2017, 2017, 5939818. [Google Scholar] [CrossRef]
- Zheng, H.J.; Guo, J.; Jia, Q.; Huang, Y.S.; Huang, W.J.; Zhang, W.; Zhang, F.; Liu, W.; Wang, Y. The effect of probiotic and synbiotic supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019, 142, 303–313. [Google Scholar] [CrossRef]
- Lin, S.W.; Tsai, Y.S.; Chen, Y.L.; Wang, M.F.; Chen, C.C.; Lin, W.H.; Fang, T.J. Lactobacillus plantarum GKM3 Promotes Longevity, Memory Retention, and Reduces Brain Oxidation Stress in SAMP8 Mice. Nutrients 2021, 13, 2860. [Google Scholar] [CrossRef]
- Liu, C.; Tseng, Y.P.; Chan, L.P.; Liang, C.H. The potential of Streptococcus thermophiles (TCI633) in the anti-aging. J. Cosmet. Dermatol. 2021. [Google Scholar] [CrossRef]
- Chaiyasut, C.; Sivamaruthi, B.S.; Kesika, P.; Khongtan, S.; Khampithum, N.; Thangaleela, S.; Peerajan, S.; Bumrungpert, A.; Chaiyasut, K.; Sirilun, S.; et al. Synbiotic Supplementation Improves Obesity Index and Metabolic Biomarkers in Thai Obese Adults: A Randomized Clinical Trial. Foods 2021, 10, 1580. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, K.R.; Kim, N.R.; Park, S.J.; Lee, M.; Kim, O.K. Combination of Bifidobacterium longum and Galacto-Oligosaccharide Protects the Skin from Photoaging. J. Med. Food 2021, 24, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, S.; Heng, X.; Chu, W. Weissella confusa CGMCC 19,308 Strain Protects Against Oxidative Stress, Increases Lifespan, and Bacterial Disease Resistance in Caenorhabditis elegans. Probiotics Antimicrob. Proteins 2021. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.W.; Tsai, Y.S.; Chen, Y.L.; Wang, M.F.; Chen, C.C.; Lin, W.H.; Fang, T.J. An Examination of Lactobacillus paracasei GKS6 and Bifidobacterium lactis GKK2 Isolated from Infant Feces in an Aged Mouse Model. Evid Based Complement. Alternat. Med. 2021, 2021, 6692363. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, H.J.; Kim, S.A.; Park, S.D.; Shim, J.J.; Lee, J.L. Exopolysaccharide from Lactobacillus plantarum HY7714 Protects against Skin Aging through Skin-Gut Axis Communication. Molecules 2021, 26, 1651. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Suk, J.; Kang, S. Effect of Lactobacillus rhAm.nosus KCTC 5033 on the Appearance of Facial Skin due to the Ingestion of Probiotics and Paraprobiotics. J. Investig. Cosmetol. 2018, 14, 287–296. [Google Scholar] [CrossRef]
- Lee, D.E.; Huh, C.-S.; Ra, J.; Choi, I.-D.; Jeong, J.-W.; Kim, S.-H.; Ryu, J.H.; Seo, Y.K.; Koh, J.S.; Lee, J.-H.; et al. Clinical Evidence of Effects of Lactobacillus plantarum HY7714 on Skin Aging: A Randomized, Double Blind, Placebo-Controlled Study. J. Microbiol. Biotechnol. 2015, 25, 2160–2168. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Cheng, L.H.; Liu, Y.W.; Jeng, O.J.; Lee, Y.K. Gerobiotics: Probiotics targeting fundamental aging processes. Biosci. Microbiota Food Health 2021, 40, 1–11. [Google Scholar] [CrossRef]
- Saito, S.; Kakizaki, N.; Okuno, A.; Maekawa, T.; Tsuji, N.M. Lactococcus lactis subsp. Cremoris C60 restores T Cell Population in Small Intestinal Lamina Propria in Aged Interleukin-18 Deficient Mice. Nutrients 2020, 12, 3287. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, Y.; Wang, X.; Yang, R.; Zhu, X.; Zhang, Y.; Chen, C.; Yuan, H.; Yang, Z.; Sun, L. Gut bacteria Akkermansia is associated with reduced risk of obesity: Evidence from the American Gut Project. Nutr. Metab. 2020, 17, 90. [Google Scholar] [CrossRef]
- Ghoneum, M.; Abdulmalek, S.; Pan, D. Reversal of age-associated oxidative stress in mice by PFT, a novel kefir product. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420950149. [Google Scholar] [CrossRef]
- Tsao, S.P.; Nurrahma, B.A.; Kumar, R.; Wu, C.H.; Yeh, T.H.; Chiu, C.C.; Lee, Y.P.; Liao, Y.C.; Huang, C.H.; Yeh, Y.T.; et al. Probiotic Enhancement of Antioxidant Capacity and Alterations of Gut Microbiota Composition in 6-Hydroxydopamin-Induced Parkinson’s Disease Rats. Antioxidants 2021, 10, 1823. [Google Scholar] [CrossRef] [PubMed]
- Lew, L.C.; Hor, Y.Y.; Jaafar, M.H.; Lau, A.S.Y.; Ong, J.S.; Chuah, L.O.; Yap, K.P.; Azzam, G.; Azlan, A.; Liong, M.T. Lactobacilli modulated AMPK activity and prevented telomere shortening in ageing rats. Benef. Microbes 2019, 10, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Freire, M.; Diaz-Ruiz, A.; Hauser, D.; Martinez-Romero, J.; Ferrucci, L.; Bernier, M.; de Cabo, R. The road ahead for health and lifespan interventions. Ageing Res. Rev. 2020, 59, 101037. [Google Scholar] [CrossRef]
- Pearson, J.A.; Wong, F.S.; Wen, L. Crosstalk between circadian rhythms and the microbiota. Immunology 2020, 161, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Kiefte-de Jong, J.C.; Mathers, J.C.; Franco, O.H. Nutrition and healthy ageing: The key ingredients. Proc. Nutr. Soc. 2014, 73, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Dominguez, L.J.; Di Bella, G.; Veronese, N.; Barbagallo, M. Impact of Mediterranean Diet on Chronic Non-Communicable Diseases and Longevity. Nutrients 2021, 13, 2028. [Google Scholar] [CrossRef] [PubMed]
- Tousian, H.; Razavi, B.M.; Hosseinzadeh, H. In search of elixir: Pharmacological agents against stem cell senescence. Iran J. Basic Med. Sci. 2021, 24, 868–880. [Google Scholar] [CrossRef]
- Hu, D.; Xie, F.; Xiao, Y.; Lu, C.; Zhong, J.; Huang, D.; Chen, J.; Wei, J.; Jiang, Y.; Zhong, T. Metformin: A Potential Candidate for Targeting Aging Mechanisms. Aging Dis. 2021, 12, 480–493. [Google Scholar] [CrossRef]
- Mohammed, I.; Hollenberg, M.D.; Ding, H.; Triggle, C.R. A Critical Review of the Evidence That Metformin Is a Putative Anti-Aging Drug That Enhances Healthspan and Extends Lifespan. Front. Endocrinol. 2021, 12, 718942. [Google Scholar] [CrossRef]
- Pryor, R.; Norvaisas, P.; Marinos, G.; Best, L.; Thingholm, L.B.; Quintaneiro, L.M.; De Haes, W.; Esser, D.; Waschina, S.; Lujan, C.; et al. Host-Microbe-Drug-Nutrient Screen Identifies Bacterial Effectors of Metformin Therapy. Cell 2019, 178, 1299–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadi, S.; Razazan, A.; Nagpal, R.; Jain, S.; Wang, B.; Mishra, S.P.; Wang, S.; Justice, J.; Ding, J.; McClain, D.A.; et al. Metformin Reduces Aging-Related Leaky Gut and Improves Cognitive Function by Beneficially Modulating Gut Microbiome/Goblet Cell/Mucin Axis. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, e9–e21. [Google Scholar] [CrossRef]
- Lee, C.B.; Chae, S.U.; Jo, S.J.; Jerng, U.M.; Bae, S.K. The Relationship between the Gut Microbiome and Metformin as a Key for Treating Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 3566. [Google Scholar] [CrossRef]
- Crimmins, E.M. Lifespan and Healthspan: Past, Present, and Promise. Gerontologist 2015, 55, 901–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beltrán-Sánchez, H.; Soneji, S.; Crimmins, E.M. Past, Present, and Future of Healthy Life Expectancy. Cold Spring Harb. Perspect. Med. 2015, 5, a025957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duar, R.M.; Henrick, B.M.; Casaburi, G.; Frese, S.A. Integrating the Ecosystem Services Framework to Define Dysbiosis of the Breastfed Infant Gut: The Role of B. infantis and Human Milk Oligosaccharides. Front. Nutr. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Coman, V.; Vodnar, D.C. Gut microbiota and old age: Modulating factors and interventions for healthy longevity. Exp. Gerontol. 2020, 141, 111095. [Google Scholar] [CrossRef] [PubMed]
- Popkes, M.; Valenzano, D.R. Microbiota-host interactions shape ageing dynamics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190596. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, S.; Sanchez-Carrillo, S.; Ciordia, S.; Mena, M.C.; Méndez-García, C.; Rojo, D.; Bargiela, R.; Zubeldia-Varela, E.; Martínez-Martínez, M.; Barbas, C.; et al. Functional microbiome deficits associated with ageing: Chronological age threshold. Aging Cell 2020, 19, e13063. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.C.N. Child modern slavery, trafficking and health: A practical review of factors contributing to children’s vulnerability and the potential impacts of severe exploitation on health. BMJ Paediatr. Open 2020, 4, e000327. [Google Scholar] [CrossRef] [PubMed]
- Franchino-Olsen, H.; Chesworth, B.R.; Boyle, C.; Rizo, C.F.; Martin, S.L.; Jordan, B.; Macy, R.J.; Stevens, L. The Prevalence of Sex Trafficking of Children and Adolescents in the United States: A Scoping Review. Trauma Violence Abus. 2022, 23, 182–195. [Google Scholar] [CrossRef]
- Reid, J.A.; Baglivio, M.T.; Piquero, A.R.; Greenwald, M.A.; Epps, N. Human Trafficking of Minors and Childhood Adversity in Florida. Am. J. Public Health 2017, 107, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Peck, J.L.; Meadows-Oliver, M.; Hays, S.M.; Maaks, D.G. White Paper: Recognizing Child Trafficking as a Critical Emerging Health Threat. J. Pediatr. Health Care 2021, 35, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Ottisova, L.; Smith, P.; Shetty, H.; Stahl, D.; Downs, J.; Oram, S. Psychological consequences of child trafficking: An historical cohort study of trafficked children in contact with secondary mental health services. PLoS ONE 2018, 13, e0192321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenbaum, J.; Bodrick, N. Committee on child abuse and neglect.; section on international child health, Global Human Trafficking and Child Victimization. Pediatrics 2017, 140, e20173138. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, S. Ghislaine Maxwell Convicted of Trafficking Girls for Jeffrey Epstein. Washington Post, 29 December 2021. Available online: https://www.washingtonpost.com/national-security/ghislaine-maxwell-verdict-guilty/2021/12/29/55b34a52-6269-11ec-a7e8-3a8455b71fad_story.html (accessed on 3 January 2022).
- Wang, Z.; Wei, L.; Peng, S.; Niu, B. Child-trafficking networks of illegal adoption in China. Nat. Sustain. 2018, 1, 254–260. [Google Scholar] [CrossRef]

| Conditions/Attribute | Details | References |
|---|---|---|
| Compromised immune system | Chronic inflammation | [23] |
| Compromised immune system | Elevated inflammation in women: Late menopause timed. | [24] |
| Compromised immune system | Early senescence in natural killer cells. | [25] |
| Elevated risk of NCDs | Elevated risk for the vast majority of the NCDs connected to ACEs. | [7,9,26,27] |
| Premature Aging/Shorter Lifespan | Epigenetics changes; Inflammaging; Shorter telomere length. | [28,29] |
| Sleep disorders | ACE-connected sleep disorders reported both in adolescents and adult; various sleep disturbances; examined via longitudinal study and other methods. | [30,31,32,33] |
| Circadian rhythm disruption | Often measured as disrupted circadian cortisol secretion or secondarily as clock gene expression. | [34,35,36] |
| Substance Misuse/Abuse/Addiction | Smoking/Alcohol/Drugs | [37,38] |
| Additional Risky Behavior | Early sex, multiple partners/early pregnancy | [39] |
| Chronic pain | Both adolescent and adult onset; cortisol levels can be a biomarker. | [40,41,42,43,44] |
| Unfavorable pregnancy outcomes | In women, one example is premature deliveries. For this outcome, a primary driver is childhood sexual abuse. | [45] |
| Neural wiring alterations/Cognitive impairment | Neural rewiring appears to be associated with a cadre of neurobehavioral alterations. | [46,47] |
| Social/Emotional Impairment | This can range from mild social interaction issues to trauma-associated body disassociation. | [48,49] |
| Elevated risk of suicide | This often co-occurs with major depressive disorder. | [50,51] |
| HPA axis dysregulation | This recent review describes a neuroimmune regulation model for the resulting HPA hyperactivity. | [52,53] |
| Complex Post-Traumatic Stress Disorder (C-PTSD). This disorder is defined in the World Health Organization International Classification of Diseases 11th Revision [54] | The C-PTSD is a specialized category of PTSD that stems from childhood trauma. | [55,56,57] |
| Microbiome dysbiosis | Dysbiosis of the gut microbiomes from ACEs appears to lock in systems biology based dysfunctional responses to later life stress. | [58,59] |
| Period of Early Life | Biomarkers (Population Subset) | NCD/CD Elevated Risk and/or Inflammation | Reference(s) |
|---|---|---|---|
| Early life adverse event | Fear as a marker of intensity | Irritable Bowel Syndrome | [63] |
| Adverse childhood event | Ace-induced immune programming appears to be a significant factor. | Pediatric Asthma | [12,64] |
| Adverse childhood experiences | Cross-sectional study demonstrating a strong positive association of developmental programming of adult asthma. | Adult-onset asthma | [65] |
| Early life bereavement | In Women | Transgenerational early onset asthma | [66] |
| Adverse childhood event | Insulin resistance; above average BMI. | Adult obesity | [67] |
| Adverse childhood event | Arterial stiffness | Cardiovascular Disease | [68] |
| Adverse childhood experiences | Female psoriasis patients had more ACEs than controls or male patients. | Psoriasis | [69] |
| Adverse childhood experiences | Evaluated in adulthood and specifically associated with other ACE comorbidities (e.g., depression). | Coronary heart disease | [70] |
| Traumatic experiences | Patients had a higher number of traumatic experiences than control groups. | Type 2 Diabetes | [71] |
| Childhood traumatic stress | Retrospective cohort study of 15,357 adults in San Diego, CA. | Elevated risk of hospitalization with adult autoimmune disease (among 21 diseases) | [72] |
| Child abuse | A study of 36,152 women from the Black Women’s Health Study. | Systemic Lupus Erythematosus | [73] |
| Child abuse | A longitudinal cohort study of 67,516 women from the Nurses’ Health Study II. | Systemic Lupus Erythematosus | [74] |
| Childhood trauma (and lifetime trauma) | A case-controlled study involving 71 HS patients and 213 controls. | Hidradenitis suppurativa (HS) | [75] |
| Early life adversity | Part of the British National child development study (NCDS), a prospective birth cohort study using 1958 births. | Cancer: elevated risk of early onset cancer (before age 50) among women | [76] |
| Adverse childhood experiences | Meta analysis for any cancer | Cancer | [16] |
| Adolescent sexual abuse | Analysis of immune parameters in patients (n = 33) with a history of childhood sexual abuse vs. controls (n = 10). | Dysregulation of the immune system (elevated eosinophils, reduced Th1 cytokines) among adolescents with PTSD | [77] |
| Childhood trauma | Results from the Netherlands Study of Depression and Anxiety (NESDA), n = 2778 | Depression and Anxiety | [78] |
| Childhood trauma | A longitudinal cohort study of 1419 British children, Adolescent inflammation was a biomarker. | Psychotic experiences | [79] |
| Adverse childhood experiences | Data analysis from the China Health and Retirement Longitudinal Study. | Adult Depression | [80] |
| Childhood trauma | Review article concluding that childhood trauma increases the risk BP. | Bipolar Disorder (BP) | [81] |
| Adverse childhood experiences | Cross sectional analysis of 1223 participants aged 65 or older. ACEs increased the likelihood of a positive diagnosis for dementia in later life. | Dementia | [82] |
| Childhood maltreatment | Case controlled study of pairs. | Endometriosis | [83] |
| Childhood trauma | A cross-sectional study involving 279 nurses from six hospitals in South Korea. | Sleep disorders | [84] |
| Adverse childhood experiences | A cross-sectional study of 22,403 adults from the 2011 Behavioral Risk Factor Surveillance System. | Short sleep duration | [31] |
| Childhood trauma | A cross sectional study from among 182 patients referred to a geriatric mental health facility. | Multidimensional frailty | [85] |
| Childhood trauma | Analysis of 655 in-patients with severe PTSD. | Post-traumatic stress disorder (PTSD) | [86] |
| Childhood trauma or high ACE score | Study of epigenetic methylation patterns of specific genes following childhood trauma as a predictor of PTSD among combat troops; 170 participants; Of seven candidate genes, three showed a lower methylation pattern associated with PTSD development following childhood trauma and/or a high ACE score. | Post-traumatic stress disor-der (PTSD) | [87] |
| Childhood trauma | Study of 155 adults with PTSD following childhood trauma; imagery rescripting (ImRs) and eye movement desensitization and reprocessing (EMDR) were found to be useful treatments of this cohort. | Post-traumatic stress disor-der (PTSD) | [88] |
| Adverse childhood experiences | Data from 108 low-income African-American adolescents; Shortened telomere length, elevated C-reactive protein levels, and increased waist circumference were all biomarkers from path analysis. | Increased cardiometabolic risk | [89] |
| Childhood maltreatment | A cross-sectional study in of 561 individuals (ranging between 6 and 14 years of age) from a large prospective community school-based study, i.e., the Brazilian High-Risk Cohort (HRC); Shortened telomere length (males only). | Shortened telomere length in males | [90] |
| Early life adversity | Study of 93 preschool-age children; early life adversity associated with increased salivary inflammatory cytokine biomarkers. | Increased salivary inflammation (based on salivary cytokine profiles) | [91] |
| Adverse childhood experiences | Analysis of 8810 members of the 1958 British birth cohort; 12 ACE criteria were used and three inflammatory markers were assessed. | Elevated inflammation was found associated with ACEs. While even some low ACE scores within specific categories of events were associated with increased inflammation in mid-life, polyadversity led to the greatest inflammation increases. Specific combination of ACEs may be more important than others pertaining to elevated inflammation. | [92] |
| Parent-child separation | 574 adolescents were evaluated. Persistent parent–child separation experiences led to significantly increased biomarkers of inflammation | Increased inflammatory burden | [93] |
| Multi-hit early life events | This is a rodent study using C3H/HeN mice of both sexes. Sex-specific effects in the adults were evident. | Microbiota alterations; Behavioral outcomes with difference in the adults based on sex. | [94] |
| Outcome(s) | Details | References |
|---|---|---|
| Pain Functional Interferences; Changes in Threat Appraisal | A study of n = 114. Males only showed significant differences. | [43] |
| Chronic pain | This was a cross-sectional analysis of the 2016–2017 National Survey of Children’s Health. There were 48,567 child participants ages 6 to 17. A significantly higher prevalence of children with one or more ACEs experienced chronic pain vs. children with no ACEs. | [96] |
| Persistent pain | This is a proposed study protocol for a systematic review and meta-analysis of ACEs and persistent pain in adults. | [40] |
| Adolescent pain | A study of 219 adolescents in rural China; Separation; host genetic variations considered; a higher pain score was found among adolescents separated from both parents. | [41] |
| Chronic Pain | A survey study of 8140 employees of City of Helsinki, Finland ages 40–60 years old. It included seven categories of ACEs. This was part of the Helsinki Health Study. | [42] |
| Cortisol levels are a useful biomarker | Review article on the social ecology of early life adversity. | [44] |
| Outcome | Details | Reference |
|---|---|---|
| Substance addiction | A qualitative study from Iceland in males who had experienced child abuse. | [110] |
| Substance misuse | Meta-analysis; A study of misuse in young people found that ACE can drive male misuse of tobacco. | [111] |
| Substance abuse | A study using data from the 2016–2019 National Survey of Children’s Health; During childhood; Seven ACEs were included (but not child maltreatment). | [46] |
| Problematic alcohol and/or tobacco use | A longitudinal study of 1179 youths of Lain American origin or descent. The authors concluded that the results suggest a predictive relationship exists for ACEs and specific abused substances. | [112] |
| Binge drinking | Data were obtained for 80,391 individuals from the Behavioral Risk Factor Surveillance System (2011–2017). Effects of combined ACEs were noted as were differential impact by sex. | [113] |
| Polysubstance use | A longitudinal study with n = 2880; Latent transition analysis was used to compare. Youths in the ACE+ group were more likely to have more categories of substance abuse and to not transit out of that as readily as ACE–youths. | [37] |
| Prescription drug misuse | A survey-based study among California college students found significant increased misuse for all prescriptions with increased odds for each added ACE. Stimulant misuse among identifying Asian/Pacific Islander (API) and Hispanic students with ACEs was noted. | [114] |
| Chronic Disorders | Reference(s) |
|---|---|
| Alcohol addiction | [123] |
| Mood disorders | [124] |
| Depression | [125] |
| Pain | [126] |
| Chronic inflammation | [127] |
| Neurodegeneration | [127] |
| Metabolic dysregulation and disease | [128,129] |
| Sleep quality/disorders | [130] |
| HPA axis dysregulation | [131] |
| Elevated risk of atherosclerosis | [132] |
| Diseases of the skin | [133] |
| Tumorigenesis | [134] |
| Increased risk of asthma | [135] |
| Promotion of allergic diseases | [136] |
| Circadian-Related Condition | Effects | Reference |
|---|---|---|
| Gut microbiota comparisons among circadian-associated sleep disruption | In this cross-sectional study, short sleepers (less than six hours) were found to have significantly lower Sutterella and significantly elevated Pseudomonas when compared with the gut microbiota of long sleepers. | [137] |
| Immune and metabolic homeostasis | A review of how cyclic metabolism of gut microbiota of short-chain fatty acids, tryptophan metabolites, and bile acids significantly affect the status of the microimmunosome (barrier function, immune cell balance, control of tolerance and inflammation). | [138] |
| Microbial oscillators of dietary cues, circadian clock, and metabolism | This review article describes how the microbiome controls chronometabolism and host metabolic phenotypes via microbial metabolites (e.g., short-chain fatty acids), microbial components (e.g., flagellin), and nuclear receptors. | [139] |
| Microbes and circadian medicine | This review focuses on (1) inherent rhythms among microbiota, (2) the cross-talk between the circadian clock and microbiota and (3) how the combined interactions either produce homeostasis or dysbiosis, immune and physiological dysfunctions and pathology. | [140] |
| Disrupted circadian rhythms | Intermittent fasting aligns circadian rhythms via the gut microbiome. | [141] |
| Circadian misalignment | Proof of concept that disrupted circacadian rhythm affects the oral microbiome composition and metabolic function as well as functional pathways affecting immunity. | [142] |
| Details | Reference(s) |
|---|---|
| A Lactobacillus plantarum probiotic was found to aid deep sleep. | [144] |
| Bifidobacterium longum supplementation provided improved sleep during heightened stress. | [145] |
| A probiotic mix improved sleep quality. | [146] |
| Gut microbiome status including altered metabolism affects sleep. | [147] |
| Probiotic supplementation improved sleep among postoperative cancer patients. | [148] |
| Sleep quality improved with a probiotic complex. | [149] |
| Specific gut microbiota predicts short vs. normal sleep. | [150] |
| Gut microbiome dysbiosis can produce an overactive bladder which disrupts sleep. | [151] |
| A review of gut microbiome status including altered metabolism and the impact on sleep. | [152] |
| A review of sleep disorders and gut dysbiosis and how they go together. | [153] |
| A review of sleep disruption and microbiome metabolic dysregulation. | [154] |
| A review of using microbiota to control the sleep–wake cycle as we age. | [155] |
| Inflammatory Damage and Telomere Status | Reference(s) |
|---|---|
| Linking telomere length, inflammation, and gut dysbiosis. | [158] |
| Oxidative stress damages telomeres and mitochondria. | [159] |
| Early life factors program both inflammation and telomere shortening. | [160] |
| Chronic inflammation generates immune aging and cross-talk with the telomere complex. | [161] |
| Link between telomere shortening and tissue inflammation. | [162] |
| Proposal that inflammation, telomere length, and microbiota may form a loop. | [163] |
| Supplement | Study/Effect | Reference(s) |
|---|---|---|
| Hyaluronic acid | Provides extracellular matrix support, acts as a form of prebiotic for gut microbiota, restores gut barrier function within the microimmunosome, acts as a therapeutic/prebiotic to rebalance skin microbiota, acts as an anti-inflammatory agent; alters macrophage polarization within the microimmunosome. | [167,168,169,170,171,172] |
| Red Ginseng | Reported dual regulation of oxidative stress and increases in Bifidobacteria and Akkermansia gut bacteria. | [173] |
| Limosilactobacillus fermentum strains | Supplementation with the probiotic mix reduced both inflammation and oxidative stress. | [174] |
| Polyphenols from Fu brick tea | Increases in core gut bacteria Akkermansia muciniphila, Alloprevotella, Bacteroides, and Faecalibaculum; improved barrier function, reduced oxidative stress in the intestine. | [175] |
| Lactobacillus salivarius FDB89; Bacillus licheniformis Lactobacillus gasseri SBT2055, Lactobacillus gasseri SBT2055, Lactococcus lactis subsp. cremoris H61, Lactococcus lactis subsp. lactis JCM 5805, Lactococcus lactis subsp. lactis strain Plasma, Lactobacillus plantarum HY7714 | A review article in 2018 listing studies with seven distinct probiotics that that were found to have anti-aging properties when administered. The first six were in model systems and the last one (Lactobacillus plantarum HY7714) was in human volunteers directed toward skin. | [176] |
| Lactobacillus fermentum DR9 Lactobacillus paracasei OFS 0291 L. helveticus OFS 1515 | Evaluation of three probiotics strains (Lactobacillus fermentum DR9, Lactobacillus paracasei OFS 0291 and L. helveticus OFS 1515 in rats for anti-aging effects in bone. Of the three, Lactobacillus fermentum DR9 was the most effective. | [177] |
| Lb. rhamnosus CRL981, Lb. plantarum CECT 748T, Lactobacillus sp. Niu-O16, Lb. rhamnosus INIA P540 Ent. faecalis INIA P333, Lb. mucosae EPI2, Ent. faecium EPI1, Finegoldia magna EPI3, and Veillonella sp. EP, Lactococcus garvieae 20–92, B. breve 15700 and B. longum BB536, B. adolescentis INIA P784, Gordonibacter urolithinfaciens and Gordonibacter pamelaeae DSM 19378T | A review of 12 different probiotic strains or mixtures that improve the senescent immune system via phytoestrogen metabolism. | [178] |
| A variety of probiotics and synbiotics among 16 studies included in this meta-analysis | A review article and meta-analysis of 16 studies of probiotics or synbiotics on diabetic patients. The results suggested that these supplements can improve biomarkers of inflammation and/or oxidative stress. | [179] |
| Lactobacillus plantarum GKM3 | A study on mice found that this probiotic promotes longevity, reduces oxidative stress in the brain, and supports memory retention. | [180] |
| Streptococcus thermophilus TCI633 | A study on humans showing the anti-aging effects of this orally administered probiotic on skin. | [181] |
| Human trial with a symbiotic preparation (Lactobacillus paracasei, Bifidobacterium longum, Bifidobacterium breve, inulin, and fructooligosaccharide) | A randomized human trial of 12 weeks duration on Thai obese adults. Among the changes seen, both inflammatory and oxidative stress biomarkers improved with the symbiotic supplement. | [182] |
| Bifidobacterium longum and the prebiotic, galacto-oligosaccharide | A study on mice found that this orally administered symbiotic combination protected against UVB-photoaging of skin. | [183] |
| Weissella confusa CGMCC 19,30 | A study on the bacterial infection C. elegans model found that this orally-administered probiotic increased lifespan, improved immunity, and reduced oxidative stress. | [184] |
| Lactobacillus paracasei GKS6 and Bifidobacterium lactis GKK2 were examined independently. | A fourteen-week study on aged mice used a two-bacteria combination probiotic to examine anti-aging effects. The results showed both probiotics significantly increased antioxidant activity thereby reducing oxidative stress. B. lactis had a positive effect on muscle building. | [185] |
| Lactobacillus plantarum HY7714 | This is a detailed mechanistic study on cell lines investigating the molecular mechanisms through which this probiotic bacterium protects skin from aging. Among the changes were reduced inflammation and oxidative stress and improved tight junction status. | [186] |
| Lactobacillus rhamnosus KCTC 5033 (a paraprobiotic group was also included in this study) | Improved skin hydration on the necks of middle-aged women following a 12-week duration trial vs. controls. | [187] |
| Lactobacillus plantarum HY7714 | A randomized, double blind, placebo-controlled study of 12 weeks duration was conducted among 100 middle-aged volunteers with dry skin. The probiotic supplementation improved skin elasticity and hydration and reduced wrinkle depth. | [188] |
| B. longum BB68, L. gasseri SBT2055, L. fermentum MBC2, B. infantis ATCC15697, B. subtilis PXN21, L. brevis OW38, L. paracasei PS23, L. paracasei K71, L. plantarum AR501, L. helveticus KLDS1.8701, L. plantarum C29, L. plantarum NDC 75017, L. fermentum DR9, B. breve B-3, L. casei Shirota | A review article including the results from 16 different probiotic strains that produce the anti-aging outcomes. The review article also proposes a new term “gerobiotics” for supplements specifically designed to produce anti-aging effects. | [189] |
| Lactococcus lactis subsp. cremoris C60 | Probiotic supplementation of IL-18 deficient mice restored a dendritic cell promoted T-cell-based immune function whose decline is connected to immune senescence. | [190] |
| Akkermansia | This survey study of the American Gut Project database confirms that Akkermansia is a major target for anti-aging protection. | [191] |
| A mixture of a specialized Lactobacillus kefiri strain product and a minor component yeast strain | A Kefir-derived product was found to reduce oxidative stress in mice. | [192] |
| L. salivarius AP-32 | Probiotic supplementation in rats increased antioxidant capacity and was neuroprotective. | [193] |
| Lactobacillus plantarum DR7, Lactobacillus fermentum DR9, Lactobacillus reuteri 8513d | Lactobaccillus probiotic strains protected against telomere shortening in a rat aging model. | [194] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dietert, R.R.; Dietert, J.M. Using Microbiome-Based Approaches to Deprogram Chronic Disorders and Extend the Healthspan following Adverse Childhood Experiences. Microorganisms 2022, 10, 229. https://doi.org/10.3390/microorganisms10020229
Dietert RR, Dietert JM. Using Microbiome-Based Approaches to Deprogram Chronic Disorders and Extend the Healthspan following Adverse Childhood Experiences. Microorganisms. 2022; 10(2):229. https://doi.org/10.3390/microorganisms10020229
Chicago/Turabian StyleDietert, Rodney R., and Janice M. Dietert. 2022. "Using Microbiome-Based Approaches to Deprogram Chronic Disorders and Extend the Healthspan following Adverse Childhood Experiences" Microorganisms 10, no. 2: 229. https://doi.org/10.3390/microorganisms10020229
APA StyleDietert, R. R., & Dietert, J. M. (2022). Using Microbiome-Based Approaches to Deprogram Chronic Disorders and Extend the Healthspan following Adverse Childhood Experiences. Microorganisms, 10(2), 229. https://doi.org/10.3390/microorganisms10020229