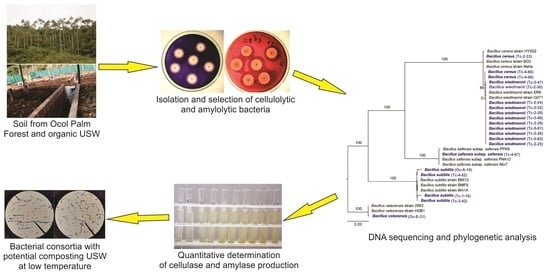
Isolation and Identification of Bacteria of Genus Bacillus from Composting Urban Solid Waste and Palm Forest in Northern Peru
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site and Study Material
2.2. Determination of pH, Temperature, Electrical Conductivity, and Humidity
2.3. Cellulolytic Capacity of Isolates
2.4. Amylolytic Capacity of Isolates
2.5. Bacterial Enzyme Activity Screening at 15 °C
2.6. Phenotypic Identification of Bacterial Isolates
2.7. DNA Sequencing and Phylogenetic Analysis of Bacterial Isolates
2.8. Bacterial Extracellular Enzyme Activities by Reducing Sugars
2.9. Analysis of Data
3. Results
3.1. Physicochemical Characterization of the Collected Samples
3.2. Bacterial Isolation and Screening for Cellulolytic and Amylolytic Activities at 30 °C and 15 °C
3.3. Bacterial Extracellular Enzyme Activity at 15 °C
3.4. Molecular Identification and Phylogenetic Analysis of Bacterial Isolates
3.5. Phenotypic Characterization of Bacterial Isolates
3.5.1. Morphological and Biochemical Analyses
3.5.2. Physiological Characterization
3.6. Bacterial Extracellular Enzyme Activity
4. Discussion
4.1. Cellulolytic and Amylolytic Activity
4.2. Molecular Identification and Phylogenetic Analysis of Bacterial Isolates
4.3. Phenotypic Identification
4.4. Bacterial Extracellular Enzyme Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahjabeen, F.; Khan, S.; Choudhury, N.; Hossain, M.M.; Khan, T.T. Isolation of Cellulolytic Bacteria from Soil, Identification by 16S rRNA Gene Sequencing and Characterization of Cellulase. Bangladesh J. Microbiol. 2016, 33, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Musa, A.M.; Ishak, C.F.; Karam, D.S.; Md Jaafar, N. Effects of fruit and vegetable wastes and biodegradable municipal wastes co-mixed composts on nitrogen dynamics in an oxisol. Agronomy 2020, 10, 1609. [Google Scholar] [CrossRef]
- Gidarakos, E.; Havas, G.; Ntzamilis, P. Municipal solid waste composition determination supporting the integrated solid waste management system in the island of Crete. Waste Manag. 2006, 26, 668–679. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Y.; Zhan, L.; Bian, X. Engineering properties for high kitchen waste content municipal solid waste. J. Rock Mech. Geotech. Eng. 2015, 7, 646–658. [Google Scholar] [CrossRef] [Green Version]
- Awasthi, M.K.; Wong, J.W.C.; Kumar, S.; Awasthi, S.K.; Wang, Q.; Wang, M.; Ren, X.; Zhao, J.; Chen, H.; Zhang, Z. Biodegradation of food waste using microbial cultures producing thermostable α-amylase and cellulase under different pH and temperature. Bioresour. Technol. 2018, 248, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Saha, A. Isolation and Characterization of Bacteria Isolated from Municipal Solid Waste for Production of Industrial Enzymes and Waste Degradation. J. Microbiol. Y Exp. 2014, 1, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Al-Dhabi, N.A.; Esmail, G.A.; Ghilan, A.K.M.; Arasu, M.A. Composting of vegetable waste using microbial consortium and biocontrol efficacy of Streptomyces sp. Al.Dhabi 30 isolated from the Saudi Arabian environment for sustainable agriculture. Sustainability 2019, 11, 6845. [Google Scholar] [CrossRef] [Green Version]
- Akintola, A.I.; Oyedeji, O.; Adewale, I.O.; Bakare, M.K. Production and physicochemical properties of thermostable, crude cellulase from Enterobacter cloacae IP8 isolated from plant leaf litters of Lagerstroemia indica linn. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 989–994. [Google Scholar] [CrossRef] [Green Version]
- De Marco, E.G.; Heck, K.; Martos, E.T.; Van Der Sand, S.T. Purification and characterization of a thermostable alkaline cellulase produced by Bacillus licheniformis 380 isolated from compost. An. Da Acad. Bras. De Ciências 2017, 89, 2359–2370. [Google Scholar] [CrossRef] [Green Version]
- Ryckeboer, J.; Mergaert, J.; Vaes, K.; Klammer, S.; De Clercq, D.; Coosemans, J.; Insam, H.; Swings, J. A survey of bacteria and fungi occurring during composting and self-heating processes. Ann. Microbiol. 2003, 53, 349–410. [Google Scholar]
- Reyes-Torres, M.; Oviedo-Ocaña, E.R.; Dominguez, I.; Komilis, D.; Sánchez, A. A systematic review on the composting of green waste: Feedstock quality and optimization strategies. Waste Manag. 2018, 77, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, S.; Guo, X.; Zhao, T.; Zhang, B. Succession and diversity of microorganisms and their association with physicochemical properties during green waste thermophilic composting. Waste Manag. 2018, 73, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Ni’matuzahroh; Affandi, M.; Fatimah; Trikurniadewi, N.; Abidin, A.Z.; Sari, S.K.; Jannah, M.; Khiftiyah, A.M. Diversity and enzymatic potential of bacteria isolated from household waste compost. AIP Conf. Proc. 2021, 2554, 090010. [Google Scholar]
- Robledo-Mahón, T.; Calvo, C.; Aranda, E. Enzymatic potential of bacteria and fungi isolates from the sewage sludge composting process. Appl. Sci. 2020, 10, 7763. [Google Scholar] [CrossRef]
- De Maayer, P.; Anderson, D.; Cary, C.; Cowan, D.A. Some like it cold: Understanding the survival strategies of psychrophiles. EMBO Rep. 2014, 15, 508–517. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, S.; Claverie, P.; Collins, T.; Georlette, D.; Gratia, E.; Hoyoux, A.; Meuwis, M.A.; Feller, G.; Gerday, C. Molecular basis of cold adaptation. Phil. Trans. R. Soc. Lond. B 2002, 357, 917–925. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Shang, X.; Luo, W.; Yuan, J.; Xu, T.; Wei, Y.; Li, J. Carbohydrates and genetic properties of two psychrophile pseudomonas B 5-16 and B 6-15. Environ. Technol. Innov. 2021, 22, 101422. [Google Scholar] [CrossRef]
- Xie, X.Y.; Zhao, Y.; Sun, Q.H.; Wang, X.Q.; Cui, H.Y.; Zhang, X.; Li, Y.J.; Wei, Z.M. A novel method for contributing to composting start-up at low temperature by inoculating cold-adapted microbial consortium. Bioresour. Technol. 2017, 238, 39–47. [Google Scholar] [CrossRef]
- Miller, R.A.; Beno, S.M.; Kent, D.J.; Carroll, L.M.; Martin, N.H.; Boor, K.J.; Kovac, J. Bacillus wiedmannii sp. nov., a psychrotolerant and cytotoxic Bacillus cereus group species isolated from dairy foods and dairy environments. Int. J. Syst. Evol. Microbiol. 2016, 66, 4744. [Google Scholar] [CrossRef]
- Sherpa, M.T.; Najar, I.N.; Das, S.; Thakur, N. Diversity of Bacillus species from Chumbu glacier. Res. J. Life Sci. Bioinf. Pharm. Chem. Sci. 2018, 4, 164–174. [Google Scholar]
- López, J.O.S.; López, R.S.; Briceño, N.B.R.; Fernández, D.G.; Murga, R.E.T.; Trigoso, D.I.; Castillo, E.B.; Cruz, M.O.; Gurbillón, M.A.B. Analytic Hierarchy Process (AHP) for a landfill site selection in Chachapoyas and Huancas (NW Peru): Modeling in a GIS-RS environment. Adv. Civ. Eng. 2022, 2022, 9733322. [Google Scholar]
- Yunis, C.R.C.; López, R.S.; Cruz, S.M.O.; Castillo, E.B.; López, J.O.S.; Tirgoso, D.I.; Briceño, N.B.R. Land suitability for sustainable aquaculture of rainbow trout (Oncorhynchus mykiss) in Molinopampa (Peru) based on RS, GIS, and AHP. Int. J. Geo-Inf. 2020, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Xu, R.; Zhang, Y.; Tang, H.; Zhou, C.; Cao, A.; Zhao, G.; Guo, H. Development of a novel compound microbial agent for degradation of kitchen waste. Braz. J. Microbiol. 2017, 48, 442–450. [Google Scholar] [CrossRef]
- Teather, R.M.; Wood, P.J. Use of Congo Red-Polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl. Environ. Microbiol. 1982, 43, 777–780. [Google Scholar] [CrossRef] [Green Version]
- Ozkan, B.M.; Ahmet, A. Isolation, identification and molecular characterization of cellulolytic bacteria from rumen samples collected from Erzurum slaughter house, Turkey. Res. J. Biotechnol. 2016, 11, 32–38. [Google Scholar]
- Ng, S.M.; Tey, L.H.; Leong, S.Y.; Ng, S.A. Isolation, screening and characterization of the potential microbes to enhance the conversion of food-wastes to bio-fertilizer. In AIP Conference Proceedings; AIP Publishing LLC: Perak, Malaysia, 2019; Volume 2157, p. 020048. [Google Scholar]
- Naresh, S.; Kunasundari, B.; Gunny, A.A.N.; Teoh, Y.P.; Shuit, S.H.; Ng, Q.H.; Hoo, P.Y. Isolation and partial characterization of thermophilic cellulolytic bacteria from north Malaysian tropical mangrove soil. Trop. Life Sci. Res. 2019, 30, 123. [Google Scholar] [CrossRef] [PubMed]
- Siu-Rodas, Y.; Calixto-Romo, M.; Guillén-Navarro, K.; Sánchez, J.E.; Zamora-Briseno, J.A.; Amaya-Delgado, L. Bacillus subtilis with endocellulase and exocellulase activities isolated in the thermophilic phase from composting with coffee residues. Rev. Argent. Microbiol. 2018, 50, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Muyzer, G.; Teske, A.; Wirsen, C.O.; Jannasch, H.W. Phylogenetic relationships of Thiomicrospira species and their identification in deep-sea hydrothermal vent samples by denaturing gradient gel electrophoresis of 16S rDNA fragments. Arch. Microbiol. 1995, 164, 165–172. [Google Scholar] [CrossRef]
- Rooney, A.P.; Price, N.P.; Ehrhardt, C.; Swezey, J.L.; Bannan, J.D. Phylogeny and molecular taxonomy of the Bacillus subtilis species complex and description of Bacillus subtilis subsp. inaquosorum subsp. nov. Int. J. Syst. Evol. Microbiol. 2009, 59, 2429–2436. [Google Scholar] [CrossRef]
- Gharsa, H.B.; Bouri, M.; Hamdane, A.M.; Schuster, C.; Leclerque, A.; Rhouma, A. Bacillus velezensis strain MBY2, a potential agent for the management of crown gall disease. PLoS ONE 2021, 16, e0252823. [Google Scholar] [CrossRef]
- McCarthy, C. Chromas, version 1.45. School of Health Science. Griffith University, Southport, Australia. Available online: http://www.technelysium.com.au/chromas.html (accessed on 14 January 2022).
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanfear, R.; Calcott, B.; Ho, S.Y.; Guindon, S. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef] [Green Version]
- Silvestro, D.; Michalak, I. raxmlGUI: A graphical front-end for RAxML. Org. Divers. Evol. 2012, 12, 335–337. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Pham, V.H.T.; Kim, J.; Shim, J.; Chang, S.; Chung, W. Coconut mesocarp-based lignocellulosic waste as a substrate for cellulase production from high promising multienzyme-producing Bacillus amyloliquefaciens FW2 without pretreatments. Microorganisms 2022, 10, 327. [Google Scholar] [CrossRef]
- Hankin, L.; Anagnostakis, S.L. The use of solid media for detection of enzyme production by fungi. Mycologia 1975, 67, 597–607. [Google Scholar] [CrossRef]
- Abd-Elhalem, B.T.; El-Sawy, M.; Gamal, R.F.; Abou-Taleb, K.A. Production of amylases from Bacillus amyloliquefaciens under submerged fermentation using some agro-industrial by-products. Annals of Agric. Sci. 2015, 60, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Olukunle, O.F.; Ayodeji, A.O.; Akinloye, P.O. Carboxymethyl Cellulase (CMCase) from UV-irradiation Mutated Bacillus cereus FOA-2 cultivated on Plantain (Musa parasidiaca) Stalk-based Medium: Production, Purification and Characterization. Sci. Afr. 2021, 11, e00691. [Google Scholar] [CrossRef]
- Da Costa Junior, J.A.; Da Rosa, G.M.; Wastowski, A.D.; Soriani, H.H.; Locatelli, A.P.C.; Da Silva, D.W.; Gonçalves, D.B.; Volpi, G.B.; Konzen, I.S.; Flach, K.A.; et al. Biotechnology: Identification and evaluation of the Bacillus cereus amylolytic activity. Res. Soc. Dev. 2021, 10, e437101321301. [Google Scholar] [CrossRef]
- Alenezi, F.N.; Slama, H.B.; Bouket, A.C.; Cherif-Silini, H.; Silini, A.; Luptakova, L.; Nowakowska, J.A.; Oszako, T.; Belbahri, L. Bacillus velezensis: A Treasure House of Bioactive Compounds of Medicinal, Biocontrol and Environmental Importance. Forests 2021, 12, 1714. [Google Scholar] [CrossRef]
- Baldwin, V.M. You Can’t B. cereus—A Review of Bacillus cereus Strains That Cause Anthrax-Like Disease. Front. Microbiol. 2020, 11, 1731. [Google Scholar] [CrossRef]
- Manzulli, V.; Rondinone, V.; Buchicchio, A.; Serrecchia, L.; Cipolletta, D.; Fasanella, A.; Parisi, A.; Difato, L.; Latarola, M.; Aceti, A. Discrimination of Bacillus cereus Group Members by MALDI-TOF Mass Spectrometry. Microorganisms 2021, 9, 1202. [Google Scholar] [CrossRef] [PubMed]
- Safitri, R.; Kusumawardhani, D.P.; Annisa, A.; Partasasmita, R.; Asharina, S.; Maskoen, A.M. Characterization and identification of three thermophilic Bacillus strain isolated from Domas Crater, Mt. Tangkuban Perahu, Indonesia. Biodiversitas J. Biol. Divers. 2020, 21, 3444–3453. [Google Scholar] [CrossRef]
- Abril, A.G.; Rama, J.L.R.; Feijoo-Siota, L.; Calo-Mata, P.; Salazar, S.; Peix, A.; Velásquez, E.; Villa, T.G. Bacillus safensis subsp. osmophilus subsp. nov., isolated from condensed milk, and description of Bacillus safensis subsp. safensis subsp. nov. Int. J. Syst. Evol. Microbiol. 2019, 69, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Shahid, M.; Noman, M.; Niazi, M.B.K.; Zubair, M.; Almatroudi, A.; Khurshid, M.; Tariq, F.; Mumtaz, R.; Li, B. Bioprospecting a native silver-resistant Bacillus safensis strain for green synthesis and subsequent antibacterial and anticancer activities of silver nanoparticles. J. Adv. Res. 2020, 24, 475–483. [Google Scholar] [CrossRef]
- Dunlap, C.A.; Bowman, M.J.; Zeigler, D.R. Promotion of Bacillus subtilis subsp. inaquosorum, Bacillus subtilis subsp. spizizenii and Bacillus subtilis subsp. stercoris to species status. Antonie Van Leeuwenhoek 2020, 113, 1–12. [Google Scholar] [CrossRef]
- Bai, S.; Kumar, M.R.; Kumar, D.J.M.; Balashanmugam, P.; Kumaran, M.D.B.; Kalaichelvan, P.T. Cellulase production by Bacillus subtilis isolated from cow dung. Sch. Res. Libr. 2012, 4, 269–279. [Google Scholar]
- Nandimath, A.P.; Kharat, K.R.; Gupta, S.G.; Kharat, A.S. Optimization of cellulase production for Bacillus sp. and Pseudomonas sp. soil isolates. Afr. J. Microbiol. Res. 2016, 10, 410–419. [Google Scholar]
- Da Silva, R.N.; Melo, L.F.A.; Finkler, C.L.L. Optimization of the cultivation conditions of Bacillus licheniformis BCLLNF-01 for cellulase production. Biotechnol. Rep. 2021, 29, e00599. [Google Scholar] [CrossRef]
- Bang, M.S.; Jeong, H.W.; Lee, Y.J.; Lee, S.C.; Lee, G.S.; Kim, S.; Lee, H.H.; Shin, J.I.; Oh, C.H. Complete Genome Sequence of Bacillus velezensis Strain DKU_NT_04, Isolated from a Traditional Korean Food Made from Soybeans (Cheonggukjang). Microbiol. Resour. Announc. 2020, 9, e00477-20. [Google Scholar] [CrossRef] [PubMed]
- Branquinho, R.; Meirinhos-Soares, L.; Carriço, J.A.; Pintado, M.; Peixe, L.V. Phylogenetic and clonality analysis of Bacillus pumilus isolates uncovered a highly heterogeneous population of different closely related species and clones. FEMS Microbiol. Ecol. 2014, 90, 689–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Onizuka, S.; Nakayama, J. Germination and cultural isolation of spore-forming bacteria in human feces by using various bile acids. Sci. Rep. 2020, 10, 15041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Diao, J.; Xie, G.; Ma, L.; Wang, L. A Complete Genome Sequence of the Wood Stem Endophyte Bacillus velezensis BY6 Strain Possessing Plant Growth-Promoting and Antifungal Activities. Biomed Res. Int. 2021, 2021, 3904120. [Google Scholar] [CrossRef] [PubMed]




| Stage/Code | Temp. °C | pH | EC mS·cm−1 | Humidity % |
|---|---|---|---|---|
| Compost samples from CMCP in Tuctilla | ||||
| Mesophilic | 29.5 | 4.07 | 1.57 | 72.4 |
| Thermophilic | 53.5 | 6.83 | 1.02 | 54.8 |
| Cooling and maturation | 36.0 | 7.04 | 1.07 | 38.0 |
| Soil samples Ocol Palm Forest | ||||
| Oc-A | 16.5 | 8.13 | 0.59 | 19.5 |
| Oc-B | 17.0 | 7.76 | 0.40 | 42.2 |
| Oc-C | 17.2 | 7.87 | 0.46 | 25.2 |
| Oc-D | 17.6 | 5.49 | 0.84 | 65.9 |
| Oc-E | 17.7 | 5.76 | 0.71 | 32.3 |
| Strain N° | Code | Specie | T (°C) | pH | NaCl (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | 60 | 5 | 6 | 7 | 8 | 9 | 10 | 5 | 10 | 20 | |||
| 1 | Tc-2-25 | B. wiedmanii | - | + | + | + | - | - | + | + | + | + | + | + | + | - | - |
| 2 | Tc-2-28 | B. wiedmanii | - | + | + | + | - | - | + | + | + | + | + | + | + | - | - |
| 3 | Tc-2-29 | B. wiedmanii | + | + | + | + | - | - | + | + | + | + | + | + | + | - | - |
| 4 | Tc-3-47 | B. wiedmanii | + | + | + | + | - | - | + | + | + | + | + | + | + | - | - |
| 5 | Tc-4-68 | B. cereus | - | + | + | + | + | - | + | + | + | + | + | + | + | - | - |
| 6 | Tc-5-82 | B. wiedmanii | - | + | + | + | - | - | + | + | + | + | + | + | + | - | - |
| 7 | Oc-A-10 | B. subtilis | - | + | + | + | + | - | + | + | + | + | + | + | + | - | - |
| 8 | Oc-E-31 | B. velezensis | + | + | + | + | + | - | + | + | + | + | + | + | + | - | - |
| 9 | Tc-2-24 | B. wiedmanii | - | + | + | + | - | - | + | + | + | + | + | + | + | - | - |
| 10 | Tc-2-30 | B. wiedmanii | - | + | + | + | - | - | + | + | + | + | + | + | + | - | - |
| 11 | Tc-2-26 | B. wiedmanii | - | + | + | + | - | - | + | + | + | + | + | + | + | - | - |
| 12 | Tc-4-66 | B. cereus | - | + | + | + | + | - | + | + | + | + | + | + | + | - | - |
| 13 | Tc-3-42 | B. subtilis | - | + | + | + | + | - | + | + | + | + | + | + | + | - | - |
| 14 | Tc-2-32 | B. wiedmanii | - | + | + | + | - | - | + | + | + | + | + | + | + | - | - |
| 15 | Tc-2-33 | B. cereus | - | + | + | + | + | - | + | + | + | + | + | + | + | - | - |
| 16 | Tc-4-67 | B. safensis subsp. safensis | + | + | + | + | + | - | + | + | + | + | + | + | + | - | - |
| 17 | Tc-1-16 | B. subtilis | - | + | + | + | + | - | + | + | + | + | + | + | + | - | - |
| 18 | Tc-3-46 | B. wiedmanii | - | + | + | + | - | - | + | + | + | + | + | + | + | - | - |
| 19 | Tc-4-62 | B. subtilis | - | + | + | + | + | - | + | + | + | + | + | + | + | - | - |
| 20 | Tc-5-81 | B. wiedmanii | + | + | + | + | - | - | + | + | + | + | + | + | + | - | - |
| Factors | Wilk’s Lambda | F | p |
|---|---|---|---|
| Strains | 0.0002 | 40.17 | ** |
| Temperature | 0.2099 | 23.53 | ** |
| Strains x temperature | 0.0190 | 7.79 | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vásquez, E.; Millones, C. Isolation and Identification of Bacteria of Genus Bacillus from Composting Urban Solid Waste and Palm Forest in Northern Peru. Microorganisms 2023, 11, 751. https://doi.org/10.3390/microorganisms11030751
Vásquez E, Millones C. Isolation and Identification of Bacteria of Genus Bacillus from Composting Urban Solid Waste and Palm Forest in Northern Peru. Microorganisms. 2023; 11(3):751. https://doi.org/10.3390/microorganisms11030751
Chicago/Turabian StyleVásquez, Ernestina, and Carlos Millones. 2023. "Isolation and Identification of Bacteria of Genus Bacillus from Composting Urban Solid Waste and Palm Forest in Northern Peru" Microorganisms 11, no. 3: 751. https://doi.org/10.3390/microorganisms11030751
APA StyleVásquez, E., & Millones, C. (2023). Isolation and Identification of Bacteria of Genus Bacillus from Composting Urban Solid Waste and Palm Forest in Northern Peru. Microorganisms, 11(3), 751. https://doi.org/10.3390/microorganisms11030751