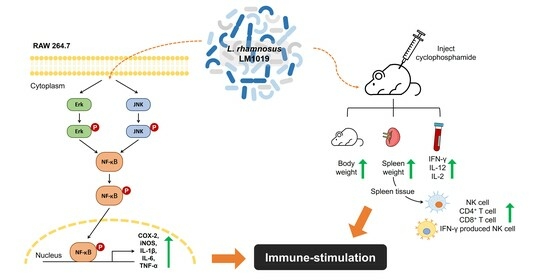
Immune-Stimulating Potential of Lacticaseibacillus rhamnosus LM1019 in RAW 264.7 Cells and Immunosuppressed Mice Induced by Cyclophosphamide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Line Experiments
2.1.1. Cell Culture
2.1.2. Cell Viability
2.1.3. The Measurement of Nitric Oxide Generation (Griess Assay)
- Stimulatory Effect Measurement: Serially diluted LM1019 doses (1 × 107, 2 × 107, 5 × 107, 1 × 108 CFU/mL) were applied to the cells to assess their stimulatory effect. To determine the relative intensity, a concentration of 10 ng/mL LPS was used as the positive control for immune stimulation.
- Inhibitory Effect Evaluation: The cells were pre-incubated with serially diluted LM1019 (106, 107, 108 CFU/mL) for 2 h, followed by the addition of LPS to each well (final concentration = 100 ng/mL) except for the negative control.
2.1.4. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.1.5. Protein Analysis
2.1.6. NF-κB Translocation
2.2. Animal Experiments
2.2.1. Animals
2.2.2. Experimental Design and LM1019 Treatment
- Normal control group (NC)
- Model control group (MC): CTX-induced immunosuppression
- CTX + beta-glucan group (PC): CTX-induced immunosuppression + beta-glucan 80 mg/kg
- CTX + LM1019 low-dose group (LM-L): CTX-induced immunosuppression + 108 CFU/head
- CTX + LM1019 high-dose group (LM-H): CTX-induced immunosuppression + 109 CFU/head
2.2.3. Change in Lymphocyte Activity
2.2.4. Serum Cytokine Level Change
2.3. Statistical Analysis
3. Results
3.1. Immune System-Stimulating Effects of LM1019 on RAW 264.7 Cells
3.1.1. LM1019-Mediated Upregulation of Nitric Oxide Production and Gene Expressions of Proinflammatory Cytokines
3.1.2. Overexpression of Proinflammatory Proteins and Signaling Pathway for Immune Stimulation by LM1019
3.2. Immune System-Stimulating Effect of LM1019 in an Immunosuppressive Mouse Model
3.2.1. The Changes in the Weights of the Body and the Spleen
3.2.2. The Changes in Lymphocyte Populations
3.2.3. Proinflammatory Cytokines in the Serums
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Perdaens, O.; van Pesch, V. Molecular Mechanisms of Immunosenescene and Inflammaging: Relevance to the Immunopathogenesis and Treatment of Multiple Sclerosis. Front. Neurol. 2022, 25, 811518. [Google Scholar] [CrossRef] [PubMed]
- Pangrazzi, L.; Weinberger, B. T cells, aging and senescence. Exp. Gerontol. 2020, 22, 110887. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular mechanisms and diseases. Signal Transduct. Target. Ther. 2023, 8, 200. [Google Scholar] [CrossRef]
- Sainz, B., Jr.; Mossel, E.C.; Peters, C.J.; Garry, R.F. Interferon-beta and interferon-gamma synergistically inhibit the replication of severe acute respiratory syndrome-associated coronavirus (SARS-CoV). Virology 2004, 329, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Finter, N.B.; Chapman, S.; Dowd, P.; Johnston, J.M.; Manna, V.; Sarantis, N.; Sheron, N.; Scott, G.; Phua, S.; Tatum, P.B. The use of interferon-alpha in virus infections. Drugs 1991, 42, 749–765. [Google Scholar] [CrossRef] [PubMed]
- Vivancos, P.; Grañena, A., Jr.; Sarrá, J.; Grañena, A. Treatment with interleukin-2 (IL-2) and interferon (IFN(alpha 2b)) after autologous bone marrow or peripheral blood stem cell transplantation in onco-hematological malignancies with a high risk of relapse. Bone Marrow Transplant. 1999, 23, 169–172. [Google Scholar] [CrossRef]
- Antoniou, K.M.; Ferdoutsis, E.; Bouros, D. Interferons and their application in the diseases of the lung. Chest 2003, 123, 209–216. [Google Scholar] [CrossRef]
- Nagpal, R.; Kumar, A.; Kumar, M.; Behare, P.V.; Jain, S.; Yadav, H. Probiotics, their health benefits and applications for developing healthier foods: A review. FEMS Microbiol. Lett. 2012, 334, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Colella, M.; Charitos, I.A.; Ballini, A.; Cafiero, C.; Topi, S.; Palmirotta, R.; Santacroce, L. Microbiota revolution: How gut microbes regulate our lives. World J. Gastroenterol. 2023, 29, 4368–4383. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Zhang, X. Probiotics Regulate Gut Microbiota: An Effective Method to Improve Immunity. Molecules 2021, 26, 6076. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.A.; Shibl, A. Role of Probiotics in health improvement, infection control and disease treatment and management. Saudi Pharm. J. 2015, 23, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Yeşilyurt, N.; Yılmaz, B.; Ağagündüz, D.; Capasso, R. Involvement of Probiotics and Postbiotics in the Immune System Modulation. Biologics 2021, 1, 89–110. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Bezirtzoglou, E. Probiotics in Medicine: A Long Debate. Front. Immunol. 2020, 11, 2192. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sood, U.; Gupta, V.; Singh, M.; Scaria, J.; Lal, R. Recent Advancements in the Development of Modern Probiotics for Restoring Human Gut Microbiome Dysbiosis. Indian J. Microbiol. 2020, 60, 12–25. [Google Scholar] [CrossRef]
- Haghighi, H.R.; Gong, J.; Gyles, C.L.; Hayes, M.A.; Zhou, H.; Sanei, B.; Chambers, J.R.; Sharif, S. Probiotics stimulate production of natural antibodies in chickens. Clin. Vaccine Immunol. 2006, 13, 975–980. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Y.; Yang, W.; Liu, Y.; Shi, C.; Liu, J.; Gao, X.; Huang, H.; Niu, T.; Yang, G.; et al. Protective effects of a food-grade recombinant Lactobacillus plantarum with surface displayed AMA1 and EtMIC2 proteins of Eimeria tenella in broiler chickens. Microb. Cell Fact. 2020, 19, 28. [Google Scholar] [CrossRef]
- Plantinga, T.S.; van Maren, W.W.; van Bergenhenegouwen, J.; Hameetman, M.; Nierkens, S.; Jacobs, C.; de Jong, D.J.; Joosten, L.A.; van’t Land, B.; Garssen, J.; et al. Differential Toll-like receptor recognition and induction of cytokine profile by Bifidobacterium breve and Lactobacillus strains of probiotics. Clin. Vaccine Immunol. 2011, 18, 621–628. [Google Scholar] [CrossRef]
- Zeuthen, L.H.; Fink, L.N.; Frøkiaer, H. Toll-like receptor 2 and nucleotide-binding oligomerization domain-2 play divergent roles in the recognition of gut-derived lactobacilli and bifidobacteria in dendritic cells. Immunology 2008, 124, 489–502. [Google Scholar] [CrossRef]
- Morata de Ambrosini, V.I.; Gonzalez, S.N.; Oliver, G. Study of adhesion of Lactobacillus casei CRL 431 to ileal intestinal cells of mice. J. Food Prot. 1999, 62, 1430–1434. [Google Scholar] [CrossRef]
- Gorreja, F.; Walker, W.A. The potential role of adherence factors in probiotic function in the gastrointestinal tract of adults and pediatrics: A narrative review of experimental and human studies. Gut Microbes 2022, 14, 2149214. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, R.; Altermann, E.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Roy, N.C. The role of cell surface architecture of lactobacilli in host-microbe interactions in the gastrointestinal tract. Mediat. Inflamm. 2013, 2013, 237921. [Google Scholar] [CrossRef] [PubMed]
- Vélez, M.P.; De Keersmaecker, S.C.; Vanderleyden, J. Adherence factors of Lactobacillus in the human gastrointestinal tract. FEMS Microbiol. Lett. 2007, 276, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Sim, J.R.; Yun, C.H.; Han, S.H. Lipoteichoic acids as a major virulence factor causing inflammatory responses via Toll-like receptor 2. Arch. Pharm. Res. 2016, 39, 1519–1529. [Google Scholar] [CrossRef]
- Long, E.M.; Millen, B.; Kubes, P.; Robbins, S.M. Lipoteichoic acid induces unique inflammatory responses when compared to other toll-like receptor 2 ligands. PLoS ONE 2009, 4, e5601. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Kang, C.H. Immunostimulatory Activity of Lactic Acid Bacteria Cell-Free Supernatants through the Activation of NF-κB and MAPK Signaling Pathways in RAW 264.7 Cells. Microorganisms 2022, 10, 2247. [Google Scholar] [CrossRef]
- Delgado, S.; Sánchez, B.; Margolles, A.; Ruas-Madiedo, P.; Ruiz, L. Molecules Produced by Probiotics and Intestinal Microorganisms with Immunomodulatory Activity. Nutrients 2020, 12, 391. [Google Scholar] [CrossRef]
- Han, S.K.; Shin, Y.J.; Lee, D.Y.; Kim, K.M.; Yang, S.J.; Kim, D.S.; Choi, J.W.; Lee, S.; Kim, D.H. Lactobacillus rhamnosus HDB1258 modulates gut microbiota-mediated immune response in mice with or without lipopolysaccharide-induced systemic inflammation. BMC Microbiol. 2021, 21, 146. [Google Scholar] [CrossRef]
- Cho, Y.H.; Oh, S.J. Casein Phosphopeptide (CPP)-Producing Activity and Proteolytic Ability by Some Lactic Acid Bacteria. Food Sci. Anim. Resour. 2010, 30, 443–448. [Google Scholar] [CrossRef]
- Park, H.S. Lactobacillus rhamnosus LM1019 Strain and Composition for Preventing and Treating Obesity or Diabetes Mellitus Comprising Same. US Patent 11,571,488 B2, 7 February 2023. [Google Scholar]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb Protoc. 2018, 6, 469–471. [Google Scholar] [CrossRef]
- Griet, M.; Zelaya, H.; Mateos, M.V.; Salva, S.; Juarez, G.E.; de Valdez, G.F.; Villena, J.; Salvador, G.A.; Rodriguez, A.V. Soluble factors from Lactobacillus reuteri CRL1098 have anti-inflammatory effects in acute lung injury induced by lipopolysaccharide in mice. PLoS ONE 2014, 9, e110027. [Google Scholar] [CrossRef] [PubMed]
- VanGuilder, H.D.; Vrana, K.E.; Freeman, W.M. Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques 2008, 44, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, U.; Squagila, N.; Boge, A.; Fung, P.A. The Simple Western™: A gel-free, blot-free, hands-free Western blotting rein-vention. Nat. Methods 2011, 8, v–vi. [Google Scholar] [CrossRef]
- Han, H.; You, Y.; Cha, S.; Ki, T.-R.; Sohn, M.; Park, J. Multi-Species Probiotic Strain Mixture Enhances Intestinal Barrier Function by Regulating Inflammation and Tight Junctions in Lipopolysaccharides Stimulated Caco-2 Cells. Microorganisms 2023, 11, 656. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawashde, F.A.; Al-Wajeeh, A.S.; Vishkaei, M.N.; Saad, H.K.M.; Johan, M.F.; Taib, W.R.W.; Ismail, I.; Al-Jamal, H.A.N. Thymoquinone Inhibits JAK/STAT and PI3K/Akt/ mTOR Signaling Pathways in MV4-11 and K562 Myeloid Leukemia Cells. Pharmaceuticals 2022, 15, 1123. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.H.; Kang, C.H.; Kang, H.; Cho, H. Immune-Enhancing Effects of Limosilactobacillus fermentum in BALB/c Mice Immunosuppressed by Cyclophosphamide. Nutrients 2023, 15, 1038. [Google Scholar] [CrossRef]
- Li, M.-Z.; Huang, X.-J.; Hu, J.-L.; Cui, S.W.; Xie, M.-Y.; Nie, S.-P. The protective effects against cyclophosphamide (CTX)-induced immunosuppression of three glucomannans. Food Hydrocoll. 2020, 100, 105445. [Google Scholar] [CrossRef]
- Yan, H.; Lu, J.; Wang, J.; Chen, L.; Wang, Y.; Li, L.; Miao, L.; Zhang, H. Prevention of Cyclophosphamide-Induced Immuno-suppression in Mice with Traditional Chinese Medicine Xuanfei Baidu Decoction. Front. Pharmacol. 2021, 12, 730567. [Google Scholar] [CrossRef]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef]
- Chiou, W.F.; Chen, C.F.; Lin, J.J. Mechanisms of suppression of inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells by andrographolide. Br. J. Pharmacol. 2009, 129, 1553–1560. [Google Scholar] [CrossRef]
- Liu, B.; Qu, L.; Yan, S. Cyclooxygenase-2 promotes tumor growth and suppresses tumor immunity. Cancer Cell Int. 2015, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.; Vale, V.L.C.; Silva, M.C.; Araújo, I.B.O.; Trindade, S.C.; de Moura-Costa, L.F.; Rodrigues, G.C.; Sales, T.S.; dos Santos, H.A.; de Carvalho-Filho, P.C.; et al. MAPK involvement in cytokine production in response to Corynebacterium pseudotuberculosis infection. BMC Microbiol. 2014, 14, 230. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Gao, H.; Chen, S.; Wang, Q.; Li, X.; Du, L.J.; Li, J.; Luo, Y.Y.; Li, J.X.; Zhao, L.C.; et al. Procyanidin A1 Alleviates Inflammatory Response induced by LPS through NF-κB, MAPK, and Nrf2/HO-1 Pathways in RAW264.7 cells. Sci. Rep. 2019, 9, 15087. [Google Scholar] [CrossRef]
- Kong, F.; Lee, B.H.; Wei, K. 5-Hydroxymethylfurfural Mitigates Lipopolysaccharide-Stimulated Inflammation via Suppression of MAPK, NF-κB and mTOR Activation in RAW 264.7 Cells. Molecules 2019, 24, 275. [Google Scholar] [CrossRef]
- Kim, H.S.; Hong, J.T.; Kim, Y.S.; Han, S.B. Stimulatory Effect of β-glucans on Immune Cells. Immune Netw. 2011, 11, 191–195. [Google Scholar] [CrossRef]
- Castro, E.D.M.; Calder, P.C.; Roche, H.M. β-1,3/1,6-Glucans and Immunity: State of the Art and Future Directions. Mol. Nutr. Food Res. 2020, 65, 1901071. [Google Scholar] [CrossRef]
- Chan, G.C.F.; Cahn, W.K.; Sze, D.M.Y. The effects of β-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009, 2, 25. [Google Scholar] [CrossRef]
- Graaff, P.; Govers, C.; Wichers, H.J.; Debets, R. Consumption of β-glucans to spice up T cell treatment of tumors: A review. Expert Opin. Biol. Ther. 2018, 18, 1023–1040. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, T.; Liu, C.; Shan, X.; Xu, Z.; Hong, H.; Lin, H.; Chen, J.; Sun, H. Cyclophosphamide induced physiological and biochemical changes in mice with an emphasis on sensitivity analysis. Ecotoxicol. Environ. Saf. 2021, 211, 111889. [Google Scholar] [CrossRef]
- Mukherjee, N.; Pal Choudhuri, S.; Delay, R.J.; Delay, E.R. Cellular mechanisms of cyclophosphamide-induced taste loss in mice. PLoS ONE 2017, 12, e0185473. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; van Bergenhenegouwen, J.; van de Kant, H.J.G.; Folkerts, G.; Garssen, J.; Vos, A.P.; Morgan, M.E.; Kraneveld, A.D. Specific probiotic dietary supplementation leads to different effects during remission and relapse in murine chronic colitis. Benef. Microbes 2016, 7, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Fong, F.L.Y.; Kirjavainen, P.; Wong, V.H.Y.; El-Nezami, H. Immunomodulatory effects of Lactobacillus rhamnosus GG on dendritic cells, macrophages and monocytes from healthy donors. J. Funct. Foods 2015, 13, 71–79. [Google Scholar] [CrossRef]
- Kolling, Y.; Salva, S.; Villena, J.; Alvarez, S. Are the immunomodulatory properties of Lactobacillus rhamnosus CRL1505 peptidoglycan common for all Lactobacilli during respiratory infection in malnourished mice? PLoS ONE 2018, 13, e0194034. [Google Scholar] [CrossRef]
- Kim, W.K.; Min, S.G.; Kwon, H.; Park, S.; Jo, M.J.; Ko, G. Lactobacillus rhamnosus KBL2290 Ameliorates Gut Inflammation in a Mouse Model of Dextran Sulfate Sodium-Induced Colitis. J. Microbiol. 2023, 61, 673–682. [Google Scholar] [CrossRef]
- Smout, J.; Valentin, C.; Delbauve, S.; Pauwels, J.; Köhler, A.; Flamand, V. Maternal Lactobacillus rhamnosus administration impacts neonatal CD4 T-cell activation and prevents murine T helper 2-type allergic airways disease. Front. Immunol. 2023, 13, 1082648. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, S.; Edalatian Dovom, M.R.; Yavarmanesh, M.; Sankian, M. Effect of Lacticaseibacillus rhamnosus and Lactiplantibacillus plantarum isolated from food and human origin on reduction of IgE-dependent hypersensitivity in Balb/c mice. Immunobiology 2022, 227, 152292. [Google Scholar] [CrossRef]
- Voo, P.Y.; Wu, C.T.; Sun, H.L.; Ko, J.L.; Lue, K.H. Effect of combination treatment with Lactobacillus rhamnosus and corticosteroid in reducing airway inflammation in a mouse asthma model. J. Microbiol. Immunol. Infect. 2022, 55, 766–776. [Google Scholar] [CrossRef]
- Liu, J.; Feng, X.; Li, B.; Sun, Y.; Jin, T.; Feng, M.; Ni, Y.; Liu, M. Lactobacillus rhamnosus GR-1 Alleviates Escherichia coli-Induced Inflammation via NF-κB and MAPKs Signaling in Bovine Endometrial Epithelial Cells. Front. Cell. Infect. Microbiol. 2022, 12, 809674. [Google Scholar] [CrossRef]
- Kim, K.M.; Song, J.W.; Yang, S.J.; Choi, J.W.; Sohn, J.; Han, S.K.; Shin, Y.J.; Lee, D.Y.; Lee, S.; Kim, D.H. Gut Microbiota-Mediated Immunomodulatory Effects of Lactobacillus rhamnosus HDB1258 Cultured in the Lava Seawater in the Colitis Mouse Model. J. Med. Food 2021, 24, 1169–1171. [Google Scholar] [CrossRef]
- Gupta, T.; Kaur, H.; Kapila, S.; Kapila, R. Potential probiotic Lacticaseibacillus rhamnosus MTCC-5897 attenuates Escherichia coli induced inflammatory response in intestinal cells. Arch. Microbiol. 2021, 203, 5703–5713. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Rowland, I.; Yaqoob, P. Comparative effects of six probiotic strains on immune function in vitro. Br. J. Nutr. 2012, 108, 459–470. [Google Scholar] [CrossRef] [PubMed]
- López, P.; Gueimonde, M.; Margolles, A.; Suárez, A. Distinct Bifidobacterium strains drive different immune responses in vitro. Int. J. Food Microbiol. 2010, 138, 157–165. [Google Scholar] [CrossRef]
- Villena, J.; Kitazawa, H. Modulation of intestinal TLR4-inflammatory signaling pathways by probiotic microorganisms: Lessons learned from Lactobacillus jensenii TL2937. Front. Immunol. 2014, 4, 512. [Google Scholar] [CrossRef]
- Kim, E.; Kang, Y.G.; Kim, J.H.; Kim, Y.J.; Lee, T.R.; Lee, J.; Kim, D.; Cho, J.Y. The Antioxidant and Anti-Inflammatory Activities of 8-Hydroxydaidzein (8-HD) in Activated Macrophage-Like RAW264.7 Cells. Int. J. Mol. Sci. 2018, 19, 1828. [Google Scholar] [CrossRef]
- Creagh, E.M.; O’Neill, L.A. TLRs, NLRs and RLRs: A trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006, 27, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, S.; Lun, J.; Gao, J.; Gao, X.; Gong, Z.; Wan, Y.; He, X.; Cao, H. Inhibitory Effects of the Lactobacillus rhamnosus GG Effector Protein HM0539 on Inflammatory Response Through the TLR4/MyD88/NF-κB Axis. Front. Immunol. 2020, 11, 551449. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Jang, S.; Jung, B.J.; Jang, K.S.; Kim, B.G.; Chung, D.K.; Kim, H. Differential immune-stimulatory effects of LTAs from different lactic acid bacteria via MAPK signaling pathway in RAW 264.7 cells. Immunobiology 2015, 220, 460–466. [Google Scholar] [CrossRef]
- Foligne, B.; Zoumpopoulou, G.; Dewulf, J.; Younes, A.B.; Chareyre, F.; Sirard, J.C.; Pot, B.; Grangette, C. A Key Role of Dendritic Cells in Probiotic Functionality. PLoS ONE 2007, 2, e313. [Google Scholar] [CrossRef]
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef]
- Mebius, R.; Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Martinvalet, D. ROS signaling during granzyme B-mediated apoptosis. Mol. Cell. Oncol. 2015, 2, e992639. [Google Scholar] [CrossRef] [PubMed]
- Sutton, V.R.; Davis, J.E.; Cancilla, M.; Johnstone, R.W.; Ruefli, A.A.; Sedelies, K.; Browne, K.A.; Trapani, J.A. Initiation of apoptosis by granzyme B requires direct cleavage of bid, but not direct granzyme B-mediated caspase activation. J. Exp. Med. 2000, 192, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Antonangeli, F.; Zingoni, A.; Soriani, A.; Santoni, A. Senescent cells: Living or dying is a matter of NK cells. J. Leukoc. Biol. 2019, 105, 1275–1283. [Google Scholar] [CrossRef]
- Elenkov, I.J.; Chrousos, G.P. Stress Hormones, Th1/Th2 patterns, Pro/Anti-inflammatory Cytokines and Susceptibility to Disease. Trends Endocrinol. Metab. 1999, 10, 359–368. [Google Scholar] [CrossRef]
- Kubo, M.; Hanada, T.; Yoshimura, A. Suppressors of cytokine signaling and immunity. Nat. Immunol. 2003, 4, 1169–1176. [Google Scholar] [CrossRef]






| Gene Name | Accession No. | Nucleotide Sequence | Product Size (bp) |
|---|---|---|---|
| IL-1β | NM_008361.4 | F: GGG CCT CAA AGG AAA GAA TC R: TAC CAG TTG GGG AAC TCT GC | 183 |
| IL-6 | NM_031168.2 | F: AGT TGC CTT CTT GGG ACT GA R: CAG AAT TGC CAT TGC ACA AC | 191 |
| TNF-α | D84199.2 | F: ATG AGC ACA GAA AGC ATG ATC R: TAC AGG CTT GTC ACT CGA ATT | 276 |
| iNOS | BC062378.1 | F: TTC CAG AAT CCC TGG ACA AG R: TGG TCA AAC TCT TGG GGT TC | 180 |
| COX-2 | NM_011198.4 | F: AGA AGG AAA TGG CTG CAG AA R: GCT CGG CTT CCA GTA TTG AG | 194 |
| β-Actin | NM_007393.5 | F: CCA CAG CTG AGA GGG AAA TC R: AAG GAA GGC TGG AAA AGA GC | 193 |
| Name of Antibody | Company | cat. no. | Source | Dilution |
|---|---|---|---|---|
| COX-2 | Cell Signaling Technology Inc. (Danvers, MA, USA) | 2282 | Rabbit | 1:10 |
| iNOS | 13120 | Rabbit | 1:10 | |
| p-Erk1/2 | 9101 | Rabbit | 1:10 | |
| Erk1/2 | 9102 | Rabbit | 1:10 | |
| p-JNK | 9251 | Rabbit | 1:10 | |
| JNK | 9252 | Rabbit | 1:10 | |
| GAPDH | 2118 | Rabbit | 1:50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, Y.; Kim, S.-H.; Kim, C.-H.; Kim, I.-H.; Shin, Y.; Kim, T.-R.; Sohn, M.; Park, J. Immune-Stimulating Potential of Lacticaseibacillus rhamnosus LM1019 in RAW 264.7 Cells and Immunosuppressed Mice Induced by Cyclophosphamide. Microorganisms 2023, 11, 2312. https://doi.org/10.3390/microorganisms11092312
You Y, Kim S-H, Kim C-H, Kim I-H, Shin Y, Kim T-R, Sohn M, Park J. Immune-Stimulating Potential of Lacticaseibacillus rhamnosus LM1019 in RAW 264.7 Cells and Immunosuppressed Mice Induced by Cyclophosphamide. Microorganisms. 2023; 11(9):2312. https://doi.org/10.3390/microorganisms11092312
Chicago/Turabian StyleYou, Yeji, Sung-Hwan Kim, Chul-Hong Kim, In-Hwan Kim, YoungSup Shin, Tae-Rahk Kim, Minn Sohn, and Jeseong Park. 2023. "Immune-Stimulating Potential of Lacticaseibacillus rhamnosus LM1019 in RAW 264.7 Cells and Immunosuppressed Mice Induced by Cyclophosphamide" Microorganisms 11, no. 9: 2312. https://doi.org/10.3390/microorganisms11092312
APA StyleYou, Y., Kim, S. -H., Kim, C. -H., Kim, I. -H., Shin, Y., Kim, T. -R., Sohn, M., & Park, J. (2023). Immune-Stimulating Potential of Lacticaseibacillus rhamnosus LM1019 in RAW 264.7 Cells and Immunosuppressed Mice Induced by Cyclophosphamide. Microorganisms, 11(9), 2312. https://doi.org/10.3390/microorganisms11092312