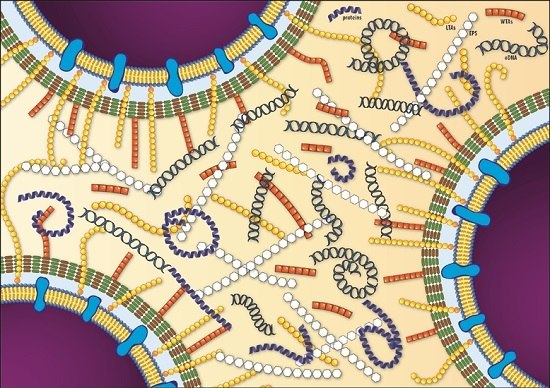
A Look inside the Listeria monocytogenes Biofilms Extracellular Matrix
Abstract
:1. Introduction
2. Listeria monocytogenes and Biofilms
3. The L. monocytogenes Biofilm Extracellular Matrix
3.1. Exopolysaccharides and Teichoic Acids
3.2. Extracellular and Biofilm-Associated Surface Proteins
3.3. Extracellular DNA
4. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BHI | brain heart infusion |
| CDC | Centers for Disease Control and Prevention |
| c-di-GMP | Cylic diguanosine monophosphate |
| CLSM | confocal laser scanning microscopy |
| ECM | extracellular matrix |
| EFSA | European Food Safety Authority |
| EPS’s | extracellular polymeric substances |
| GS-MS | gas chromatography-mass spectrometry |
| HGT | horizontal gene transfer |
| L. monocytogenes | Listeria monocytogenes |
| LTAs | lipoteichoic acids |
| NMR | nuclear magnetic resonance |
| PVC | polyvinyl chloride |
| SEM | scanning electron microscopy |
| TAs | teichoic acids |
| WTAs | wall teichoic acids |
References
- Ivanek, R.; Gröhn, Y.T.; Wiedmann, M. Listeria monocytogenes in multiple habitats and host populations: Review of available data for mathematical modeling. Foodborne Pathog. Dis. 2006, 3, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Rocourt, J.; Cossart, P. Listeria monocytogenes . In Food Microbiology—Fundamentals and Frontiers; Doyle, M.P., Buechat, L.R., Montville, T.J., Eds.; American Society for Microbiology (ASM) Press: Washington, DC, USA, 1997; pp. 337–352. [Google Scholar]
- Schirm, M.; Kalmokoff, M.; Aubry, A.; Thibault, P.; Sandoz, M.; Logan, S.M. Flagellin from Listeria monocytogenes is glycosylated with beta-O-linked N-acetylglucosamine. J. Bacteriol. 2004, 186, 6721–6727. [Google Scholar] [CrossRef] [PubMed]
- Schlech, W.F., 3rd; Lavigne, P.M.; Bortolussi, R.A.; Allen, A.C.; Haldane, E.V.; Wort, A.J.; Hightower, A.W.; Johnson, S.E.; King, S.H.; Nicholls, E.S.; et al. Epidemic listeriosis—Evidence for transmission by food. N. Engl. J. Med. 1983, 308, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the united states-major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Food- and Waterborne Diseases and Zoonoses; ECDC: Stockholm, Sweden, 2014. [Google Scholar]
- EFSA. The european union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA J. 2015, 13, 165. [Google Scholar]
- CDC. Incidence and trends of infection with pathogens transmitted commonly through food-foodborne diseases active surveillance network, 10 US sites, 2006–2013. Morb. Mortal. Wkly. Rep. 2014, 63, 328–332. [Google Scholar]
- De Noordhout, C.M.; Devleesschauwer, B.; Angulo, F.J.; Verbeke, G.; Haagsma, J.; Kirk, M.; Havelaar, A.; Speybroeck, N. The global burden of listeriosis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 1073–1082. [Google Scholar] [CrossRef]
- EFSA. The european union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2014. EFSA J. 2015, 13, 191. [Google Scholar]
- CDC. Foodborne Diseases Active Surveillance Network (Foodnet): Foodnet Surveillance Report for 2012 (Final Report); U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2014.
- Silk, B.J.; Date, K.A.; Jackson, K.A.; Pouillot, R.; Holt, K.G.; Graves, L.M.; Ong, K.L.; Hurd, S.; Meyer, R.; Marcus, R.; et al. Invasive listeriosis in the foodborne diseases active surveillance network (foodnet), 2004–2009: Further targeted prevention needed for higher-risk groups. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012, 54 (Suppl. 5), S396–S404. [Google Scholar] [CrossRef] [PubMed]
- Painter, J.; Slutsker, L. Listeriosis in humans. In Listeria, Listeriosis and Food Safety, 3rd ed.; CRC press: Boca Raton, FL, USA, 2007; pp. 85–109. [Google Scholar]
- Jackson, K.A.; Iwamoto, M.; Swerdlow, D. Pregnancy-associated listeriosis. Epidemiol. Infect. 2010, 138, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Riedo, F.X.; Pinner, R.W.; Tosca, M.L.; Cartter, M.L.; Graves, L.M.; Reeves, M.W.; Weaver, R.E.; Plikaytis, B.D.; Broome, C.V. A point-source foodborne listeriosis outbreak: Documented incubation period and possible mild illness. J. Infect. Dis. 1994, 170, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Dalton, C.B.; Austin, C.C.; Sobel, J.; Hayes, P.S.; Bibb, W.F.; Graves, L.M.; Swaminathan, B.; Proctor, M.E.; Griffin, P.M. An outbreak of gastroenteritis and fever due to Listeria monocytogenes in milk. N. Engl. J. Med. 1997, 336, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, L. Listeriosis. Pediatr. Rev. Am. Acad. Pediatr. 2008, 29, 410–411. [Google Scholar] [CrossRef] [PubMed]
- Allerberger, F. Listeria. In Foodborne Diseases; Simjee, S., Ed.; Humana Press: Totowa, NJ, USA, 2007; pp. 27–39. [Google Scholar]
- Farber, J.M.; Peterkin, P.I. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 1991, 55, 476–511. [Google Scholar] [PubMed]
- Vazquez-Boland, J.A.; Kuhn, M.; Berche, P.; Chakraborty, T.; Dominguez-Bernal, G.; Goebel, W.; Gonzalez-Zorn, B.; Wehland, J.; Kreft, J. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 2001, 14, 584–640. [Google Scholar] [CrossRef] [PubMed]
- Blackman, I.C.; Frank, J.F. Growth of Listeria monocytogenes as a biofilm on various food-processing surfaces. J. Food Prot. 1996, 59, 827–831. [Google Scholar]
- Di Bonaventura, G.; Piccolomini, R.; Paludi, D.; D’Orio, V.; Vergara, A.; Conter, M.; Ianieri, A. Influence of temperature on biofilm formation by Listeria monocytogenes on various food-contact surfaces: Relationship with motility and cell surface hydrophobicity. J. Appl. Microbiol. 2008, 104, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Mafu, A.A.; Roy, D.; Goulet, J.; Savoie, L. Characterization of physicochemical forces involved in adhesion of Listeria monocytogenes to surfaces. Appl. Environ. Microbiol. 1991, 57, 1969–1973. [Google Scholar] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.W. The biofilm matrix—An immobilized but dynamic microbial environment. TIM 2001, 9, 222–227. [Google Scholar] [CrossRef]
- Ma, L.; Wang, J.; Wang, S.; Anderson, E.M.; Lam, J.S.; Parsek, M.R.; Wozniak, D.J. Synthesis of multiple Pseudomonas aeruginosa biofilm matrix exopolysaccharides is post-transcriptionally regulated. Environ. Microbiol. 2012, 14, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Schaudinn, C.; Stoodley, P.; Kainović, A.; O’Keeffe, T.; Costerton, B.; Robinson, D.; Baum, M.; Ehrlich, G.; Webster, P. Bacterial biofilms, other structures seen as mainstream concepts. Microbe 2007, 2, 231–237. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 207–228. [Google Scholar] [PubMed]
- Berk, V.; Fong, J.C.N.; Dempsey, G.T.; Develioglu, O.N.; Zhuang, X.; Liphardt, J.; Yildiz, F.H.; Chu, S. Molecular architecture and assembly principles of Vibrio cholerae biofilms. Science 2012, 337, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Lunden, J.M.; Miettinen, M.K.; Autio, T.J.; Korkeala, H.J. Persistent Listeria monocytogenes strains show enhanced adherence to food contact surface after short contact times. J. Food Prot. 2000, 63, 1204–1207. [Google Scholar] [PubMed]
- Trémoulet, F.; Duché, O.; Namane, A.; Martinie, B.; Labadie, J.C. Comparison of protein patterns of Listeria monocytogenes grown in biofilm or in planktonic mode by proteomic analysis. FEMS Microbiol. Lett. 2002, 210, 25–31. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, B.; Cerf, O. Review—Persistence of Listeria monocytogenes in food industry equipment and premises. Int. J. Food Microbiol. 2011, 145, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Borucki, M.K.; Peppin, J.D.; White, D.; Loge, F.; Call, D.R. Variation in biofilm formation among strains of Listeria monocytogenes. Appl. Environ. Microbiol. 2003, 69, 7336–7342. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, D.; Wiedmann, M.; McLandsborough, L.A. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 2002, 68, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
- Mai, T.L.; Conner, D.E. Effect of temperature and growth media on the attachment of Listeria monocytogenes to stainless steel. Int. J. Food Microbiol. 2007, 120, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Moltz, A.G.; Martin, S.E. Formation of biofilms by Listeria monocytogenes under various growth conditions. J. Food Prot. 2005, 68, 92–97. [Google Scholar] [PubMed]
- Chae, M.S.; Schraft, H. Comparative evaluation of adhesion and biofilm formation of different Listeria monocytogenes strains. Int. J. Food Microbiol. 2000, 62, 103–111. [Google Scholar] [CrossRef]
- Tresse, O.; Shannon, K.; Pinon, A.; Malle, P.; Vialette, M.; Midelet-Bourdin, G. Variable adhesion of Listeria monocytogenes isolates from food-processing facilities and clinical cases to inert surfaces. J. Food Prot. 2007, 70, 1569–1578. [Google Scholar] [PubMed]
- Harvey, J.; Keenan, K.P.; Gilmour, A. Assessing biofilm formation by Listeria monocytogenes strains. Food Microbiol. 2007, 24, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Midelet, G.; Carpentier, B. Impact of cleaning and disinfection agents on biofilm structure and on microbial transfer to a solid model food. J. Appl. Microbiol. 2004, 97, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Midelet, G.; Kobilinsky, A.; Carpentier, B. Construction and analysis of fractional multifactorial designs to study attachment strength and transfer of Listeria monocytogenes from pure or mixed biofilms after contact with a solid model food. Appl. Environ. Microbiol. 2006, 72, 2313–2321. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Wolfaardt, G.M.; Arts, M.T. Characterization of Pseudomonas aeruginosa fatty acid profiles in biofilms and batch planktonic cultures. Can. J. Microbiol. 2010, 56, 1028–1039. [Google Scholar] [CrossRef] [PubMed]
- Chihib, N.E.; da Silva, M.R.; Delattre, G.; Laroche, M.; Federighi, M. Different cellular fatty acid pattern behaviours of two strains of Listeria monocytogenes Scott A and CNL 895807 under different temperature and salinity conditions. FEMS Microbiol. Lett. 2003, 218, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Perez, L.J.; Ng, W.L.; Marano, P.; Brook, K.; Bassler, B.L.; Semmelhack, M.F. Role of the CAI-1 fatty acid tail in the Vibrio cholerae quorum sensing response. J. Med. Chem. 2012, 55, 9669–9681. [Google Scholar] [CrossRef] [PubMed]
- Renier, S.; Hébraud, M.; Desvaux, M. Molecular biology of surface colonization by Listeria monocytogenes: An additional facet of an opportunistic Gram-positive foodborne pathogen. Environ. Microbiol. 2011, 13, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Chavant, P.; Martinie, B.; Meylheuc, T.; Bellon-Fontaine, M.N.; Hebraud, M. Listeria monocytogenes LO28: Surface physicochemical properties and ability to form biofilms at different temperatures and growth phases. Appl. Environ. Microbiol. 2002, 68, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Marsh, E.J.; Luo, H.; Wang, H. A three-tiered approach to differentiate Listeria monocytogenes biofilm-forming abilities. FEMS Microbiol. Lett. 2003, 228, 203–210. [Google Scholar] [CrossRef]
- Rieu, A.; Briandet, R.; Habimana, O.; Garmyn, D.; Guzzo, J.; Piveteau, P. Listeria monocytogenes EGD-e biofilms: No mushrooms but a network of knitted chains. Appl. Environ. Microbiol. 2008, 74, 4491–4497. [Google Scholar] [CrossRef] [PubMed]
- Guilbaud, M.; Piveteau, P.; Desvaux, M.; Brisse, S.; Briandet, R. Exploring the diversity of Listeria monocytogenes biofilm architecture by high-throughput confocal laser scanning microscopy and the predominance of the honeycomb-like morphotype. Appl. Environ. Microbiol. 2015, 81, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Bridier, A.; Dubois-Brissonnet, F.; Boubetra, A.; Thomas, V.; Briandet, R. The biofilm architecture of sixty opportunistic pathogens deciphered using a high throughput CLSM method. J. Microbiol. Methods 2010, 82, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Hancock, L.E.; Perego, M. The enterococcus faecalis fsr two-component system controls biofilm development through production of gelatinase. J. Bacteriol. 2004, 186, 5629–5639. [Google Scholar] [CrossRef] [PubMed]
- Zameer, F.; Gopal, S.; Krohne, G.; Kreft, J. Development of a biofilm model for Listeria monocytogenes EGD-e. World J. Microbiol. Biotechnol. 2010, 26, 1143–1147. [Google Scholar] [CrossRef]
- Chen, L.H.; Köseoglu, V.K.; Guvener, Z.T.; Myers-Morales, T.; Reed, J.M.; D’Orazio, S.E.; Miller, K.W.; Gomelsky, M. Cyclic di-GMP-dependent signaling pathways in the pathogenic firmicute Listeria monocytogenes. PLoS Pathog. 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Tiensuu, T.; Andersson, C.; Rydén, P.; Johansson, J. Cycles of light and dark co-ordinate reversible colony differentiation in Listeria monocytogenes. Mol. Microbiol. 2013, 87, 909–924. [Google Scholar] [CrossRef] [PubMed]
- Hefford, M.A.; D’Aoust, S.; Cyr, T.D.; Austin, J.W.; Sanders, G.; Kheradpir, E.; Kalmokoff, M.L. Proteomic and microscopic analysis of biofilms formed by Listeria monocytogenes 568. Can. J. Microbiol. 2005, 51, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Cywes-Bentley, C.; Skurnik, D.; Zaidi, T.; Roux, D.; DeOliveira, R.B.; Garrett, W.S.; Lu, X.; O’Malley, J.; Kinzel, K.; Zaidi, T.; et al. Antibody to a conserved antigenic target is protective against diverse prokaryotic and eukaryotic pathogens. Proc. Natl. Acad. Sci. USA 2013, 110, E2209–E2218. [Google Scholar] [CrossRef] [PubMed]
- Combrouse, T.; Sadovskaya, I.; Faille, C.; Kol, O.; Guérardel, Y.; Midelet-Bourdin, G. Quantification of the extracellular matrix of the Listeria monocytogenes biofilms of different phylogenic lineages with optimization of culture conditions. J. Appl. Microbiol. 2013, 114, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Brauge, T.; Sadovskaya, I.; Faille, C.; Benezech, T.; Maes, E.; Guerardel, Y.; Midelet-Bourdin, G. Teichoic acid is the major polysaccharide present in the Listeria monocytogenes biofilm matrix. FEMS Microbiol. Lett. 2016, 363. [Google Scholar] [CrossRef] [PubMed]
- Koseoglu, V.K.; Heiss, C.; Azadi, P.; Topchiy, E.; Guvener, Z.T.; Lehmann, T.E.; Miller, K.W.; Gomelsky, M. Listeria monocytogenes exopolysaccharide: Origin, structure, biosynthetic machinery and c-di-GMP-dependent regulation. Mol. Microbiol. 2015, 96, 728–743. [Google Scholar] [CrossRef] [PubMed]
- Zetzmann, M.; Okshevsky, M.; Endres, J.; Sedlag, A.; Caccia, N.; Auchter, M.; Waidmann, M.S.; Desvaux, M.; Meyer, R.L.; Riedel, C.U. Dnase-sensitive and -resistant modes of biofilm formation by Listeria monocytogenes. Front. Microbiol. 2015, 6, 1428. [Google Scholar] [CrossRef] [PubMed]
- Wingender, J.; Strathmann, M.; Rode, A.; Leis, A.; Flemming, H.C. Isolation and biochemical characterization of extracellular polymeric substances from Pseudomonas aeruginosa. Method Enzymol. 2001, 336, 302–314. [Google Scholar]
- Frolund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996, 30, 1749–1758. [Google Scholar] [CrossRef]
- Evans, L.R.; Linker, A. Production and characterization of the slime polysaccharide of Pseudomonas aeruginosa. J. Bacteriol. 1973, 116, 915–924. [Google Scholar] [PubMed]
- Sadovskaya, I.; Vinogradov, E.; Flahaut, S.; Kogan, G.; Jabbouri, S. Extracellular carbohydrate-containing polymers of a model biofilm-producing strain, Staphylococcus epidermidis rp62a. Infect. Immun. 2005, 73, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.N.; Perry, K.J.; Regeimbal, J.M.; Regan, P.M.; Higgins, D.E. Identification of Listeria monocytogenes determinants required for biofilm formation. PLoS ONE 2014, 9, e113696. [Google Scholar] [CrossRef] [PubMed]
- Longhi, C.; Scoarughi, G.L.; Poggiali, F.; Cellini, A.; Carpentieri, A.; Seganti, L.; Pucci, P.; Amoresano, A.; Cocconcelli, P.S.; Artini, M.; et al. Protease treatment affects both invasion ability and biofilm formation in Listeria monocytogenes. Microb. Pathog. 2008, 45, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, U.T.; Burrows, L.L. Dnase i and proteinase k impair Listeria monocytogenes biofilm formation and induce dispersal of pre-existing biofilms. Int. J. Food Microbiol. 2014, 187, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Franciosa, G.; Maugliani, A.; Scalfaro, C.; Floridi, F.; Aureli, P. Expression of internalin a and biofilm formation among Listeria monocytogenes clinical isolates. Int. J. Immunopathol. Pharmacol. 2009, 22, 183–193. [Google Scholar] [PubMed]
- Jordan, S.J.; Perni, S.; Glenn, S.; Fernandes, I.; Barbosa, M.; Sol, M.; Tenreiro, R.P.; Chambel, L.; Barata, B.; Zilhao, I.; et al. Listeria monocytogenes biofilm-associated protein (BapL) may contribute to surface attachment of L. monocytogenes but is absent from many field isolates. Appl. Environ. Microbiol. 2008, 74, 5451–5456. [Google Scholar] [CrossRef] [PubMed]
- Lemon, K.P.; Higgins, D.E.; Kolter, R. Flagellar motility is critical for Listeria monocytogenes biofilm formation. J. Bacteriol. 2007, 189, 4418–4424. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, A.; de Las Heras, A.; Scortti, M.; Vazquez-Boland, J.; Frank, J.F.; Brito, L. Comparison of Listeria monocytogenes exoproteomes from biofilm and planktonic state: Lmo2504, a protein associated with biofilms. Appl. Environ. Microbiol. 2013, 79, 6075–6082. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, M.; Lappann, M.; Knochel, S.; Molin, S. The role of extra-cellular DNA during biofilm formation of Listeria monocytogenes. Appl. Environ. Microbiol. 2010, 76, 3625–3636. [Google Scholar] [CrossRef] [PubMed]
- Okshevsky, M.; Meyer, R.L. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit. Rev. Microbiol. 2015, 41, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Sadovskaya, I.; Vinogradov, E.; Li, J.; Jabbouri, S. Structural elucidation of the extracellular and cell-wall teichoic acids of Staphylococcus epidermidis RP62A, a reference biofilm-positive strain. Carbohydr. Res. 2004, 339, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Whitney, J.C.; Howell, P.L. Synthase-dependent exopolysaccharide secretion in gram-negative bacteria. TIM 2013, 21, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Merighi, M.; Lee, V.T.; Hyodo, M.; Hayakawa, Y.; Lory, S. The second messenger bis-(3′-5′)-cyclic-GMP and its PilZ domain-containing receptor Alg44 are required for alginate biosynthesis in Pseudomonas aeruginosa. Mol. Microbiol. 2007, 65, 876–895. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.T.; Matewish, J.M.; Kessler, J.L.; Hyodo, M.; Hayakawa, Y.; Lory, S. A cyclic-di-GMP receptor required for bacterial exopolysaccharide production. Mol. Microbiol. 2007, 65, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.; Weinhouse, H.; Aloni, Y. Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 1987, 325, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Ryjenkov, D.A.; Simm, R.; Römling, U.; Gomelsky, M. The pilz domain is a receptor for the second messenger c-di-GMP: The PilZ domain protein ycgr controls motility in enterobacteria. J. Biol. Chem. 2006, 281, 30310–30314. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.L.W.; Strumillo, J.; Zimmer, J. Crystallographic snapshot of cellulose synthesis and membrane translocation. Nature 2013, 493, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Romling, U.; Galperin, M.Y.; Gomelsky, M. Cyclic di-GMP: The first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 2013, 77, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, C.A.; Strobel, S.A. The bacterial second messenger c-di-GMP: Probing interactions with protein and RNA binding partners using cyclic dinucleotide analogs. Org. Biomol. Chem. 2012, 10, 9113–9129. [Google Scholar] [CrossRef] [PubMed]
- Smoot, L.M.; Pierson, M.D. Influence of environmental stress on the kinetics and strength of attachment of Listeria monocytogenes Scott A to Buna-N rubber and stainless steel. J. Food Prot. 1998, 61, 1286–1292. [Google Scholar] [PubMed]
- Desvaux, M.; Dumas, E.; Chafsey, I.; Chambon, C.; Hébraud, M. Comprehensive appraisal of the extracellular proteins from a monoderm bacterium: Theoretical and empirical exoproteomes of Listeria monocytogenes egd-e by secretomics. J. Proteome Res. 2010, 9, 5076–5092. [Google Scholar] [CrossRef] [PubMed]
- Bierne, H.; Cossart, P. Listeria monocytogenes surface proteins: From genome predictions to function. Microbiol. Mol. Biol. Rev. 2007, 71, 377–397. [Google Scholar] [CrossRef] [PubMed]
- Lasa, I. Towards the identification of the common features of bacterial biofilm development. Int. Microbiol. 2006, 9, 21–28. [Google Scholar] [PubMed]
- Cucarella, C.; Solano, C.; Valle, J.; Amorena, B.; Lasa, Í.; Penadés, J.R. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 2001, 183, 2888–2896. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Arana, A.; Valle, J.; Solano, C.; Arrizubieta, M.J.; Cucarella, C.; Lamata, M.; Amorena, B.; Leiva, J.; Penades, J.R.; Lasa, I. The enterococcal surface protein, Esp, is involved in Enterococcus faecalis biofilm formation. Appl. Environ. Microbiol. 2001, 67, 4538–4545. [Google Scholar] [CrossRef] [PubMed]
- Latasa, C.; Roux, A.; Toledo-Arana, A.; Ghigo, J.M.; Gamazo, C.; Penades, J.R.; Lasa, I. Bapa, a large secreted protein required for biofilm formation and host colonization of Salmonella enterica serovar enteritidis. Mol. Microbiol. 2005, 58, 1322–1339. [Google Scholar] [CrossRef] [PubMed]
- Hinsa, S.M.; Espinosa-Urgel, M.; Ramos, J.L.; O’Toole, G.A. Transition from reversible to irreversible attachment during biofilm formation by Pseudomonas fluorescens WCS365 requires an ABC transporter and a large secreted protein. Mol. Microbiol. 2003, 49, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Todhanakasem, T.; Young, G.M. Loss of flagellum-based motility by Listeria monocytogenes results in formation of hyperbiofilms. J. Bacteriol. 2008, 190, 6030–6034. [Google Scholar] [CrossRef] [PubMed]
- Peel, M.; Donachie, W.; Shaw, A. Temperature-dependent expression of flagella of Listeria monocytogenes studied by electron microscopy, SDS-PAGE and western blotting. J. Gen. Microbiol. 1988, 134, 2171–2178. [Google Scholar] [PubMed]
- Hall-Stoodley, L.; Nistico, L.; Sambanthamoorthy, K.; Dice, B.; Nguyen, D.; Mershon, W.J.; Johnson, C.; Ze Hu, F.; Stoodley, P.; Ehrlich, G.D.; et al. Characterization of biofilm matrix, degradation by dnase treatment and evidence of capsule downregulation in Streptococcus pneumoniae clinical isolates. BMC Microbiol. 2008, 8. [Google Scholar] [CrossRef] [PubMed]
- Izano, E.A.; Amarante, M.A.; Kher, W.B.; Kaplan, J.B. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl. Environ. Microbiol. 2008, 74, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.E.; Rice, K.C.; Boles, B.R.; Endres, J.L.; Ranjit, D.; Chandramohan, L.; Tsang, L.H.; Smeltzer, M.S.; Horswill, A.R.; Bayles, K.W. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS ONE 2009, 4, e5822. [Google Scholar] [CrossRef] [PubMed]
- Seper, A.; Fengler, V.H.I.; Roier, S.; Wolinski, H.; Kohlwein, S.D.; Bishop, A.L.; Camilli, A.; Reidl, J.; Schild, S. Extracellular nucleases and extracellular DNA play important roles in Vibrio cholerae biofilm formation. Mol. Microbiol. 2011, 82, 1015–1037. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Klein, M.I.; Heim, K.P.; Fan, Y.; Bitoun, J.P.; Ahn, S.J.; Burne, R.A.; Koo, H.; Brady, L.J.; Wen, Z.T. Streptococcus mutans extracellular DNA is upregulated during growth in biofilms, actively released via membrane vesicles, and influenced by components of the protein secretion machinery. J. Bacteriol. 2014, 196, 2355–2366. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.R.; den Besten, H.M.W.; van der Veen, S.; Zwietering, M.H.; Moezelaar, R.; Abee, T. Diversity assessment of Listeria monocytogenes biofilm formation: Impact of growth condition, serotype and strain origin. Int. J. Food Microbiol. 2013, 165, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Neu, T.R.; Wozniak, D.J. The EPS matrix: The “house of biofilm cells”. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef] [PubMed]


| EPS | Molecules | Features | References |
|---|---|---|---|
| Polysaccharides | poly-β-(1,4)-N-acetylmannosamine (poly-NAM) Teichoic Acids (WTA and LTA) | - Teichoic acids are the major component of listerial biofilm matrix | [60] |
| - dltABCD mutants present a reduction in biofilm forming ability | [67] | ||
| - Biosynthesis of poly-NAM is activated by high levels of c-di-GMP | [61] | ||
| Proteins | InlA BapL PlcA FlaA PBP ActA Lmo2504 | - Proteinase K treatment impairs biofilm development, suggesting protein involvement in initial attachment | [68,69] |
| - Truncated proteins exhibited enhanced biofilm forming ability | [70] | ||
| - BapL can contribute to the attachment of some L. monocytogenes strains | [71] | ||
| - Flagellar motility has controversial role in biofilm formation | [32,51,72,73] | ||
| Extracellular DNA | - DNAseI treatment inhibited or delayed initial attachment of bacteria to surfaces, suggesting eDNA involvement in initial attachment | [69,74] | |
| - Ensure structural integrity of biofilm in cooperation with proteins and polysaccharides | [62,74,75] | ||
| - Involved in Horizontal Gene Transfer | [74] | ||
| - Serves as energy and nutrition source | [51] | ||
| - eDNA is released by lysed bacteria | [62,74] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colagiorgi, A.; Di Ciccio, P.; Zanardi, E.; Ghidini, S.; Ianieri, A. A Look inside the Listeria monocytogenes Biofilms Extracellular Matrix. Microorganisms 2016, 4, 22. https://doi.org/10.3390/microorganisms4030022
Colagiorgi A, Di Ciccio P, Zanardi E, Ghidini S, Ianieri A. A Look inside the Listeria monocytogenes Biofilms Extracellular Matrix. Microorganisms. 2016; 4(3):22. https://doi.org/10.3390/microorganisms4030022
Chicago/Turabian StyleColagiorgi, Angelo, Pierluigi Di Ciccio, Emanuela Zanardi, Sergio Ghidini, and Adriana Ianieri. 2016. "A Look inside the Listeria monocytogenes Biofilms Extracellular Matrix" Microorganisms 4, no. 3: 22. https://doi.org/10.3390/microorganisms4030022
APA StyleColagiorgi, A., Di Ciccio, P., Zanardi, E., Ghidini, S., & Ianieri, A. (2016). A Look inside the Listeria monocytogenes Biofilms Extracellular Matrix. Microorganisms, 4(3), 22. https://doi.org/10.3390/microorganisms4030022