Host–Microbe Interactions and Gut Health in Poultry—Focus on Innate Responses
Abstract
:1. Introduction
2. Inflammation: Innate Response to Noxious Stimuli
3. Relevance to Poultry Gut Health and Associated Studies?
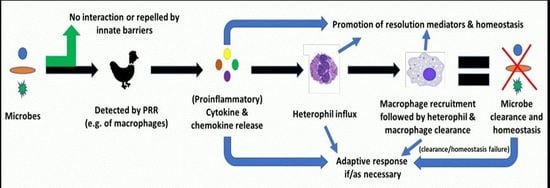
3.1. Host–Microbe Interactions
3.2. Inflammatory Responses
4. Conclusions
Funding
Conflicts of Interest
References
- Hornef, M. Pathogens, commensal symbiont, and pathobionts: Discovery and functional effects on the host. ILAR J. 2015, 56, 159–162. [Google Scholar] [CrossRef]
- Couto Garcia, C.; Guabiraba, R.; Soriani, F.M.; Teixeira, M.M. The development of anti-inflammatory drugs for infectious diseases. Discov. Med. 2010, 55, 479–488. [Google Scholar]
- Nurmi, E.; Rantala, M. New aspects of Salmonella infection in broiler production. Nature 1973, 241, 210–211. [Google Scholar] [CrossRef] [PubMed]
- Khiaosa-ard, R.; Zebeli, Q. Diet-induced inflammation: From gut to metabolic organs and the consequences for the health and longevity of ruminants. Res. Vet. Sci. 2018, 120, 17–27. [Google Scholar] [CrossRef]
- Juul-Madsen, H.R.; Viertlboeck, B.; Hartle, S.; Smith, A.L.; Gobel, T.W. Innate immune responses. In Avian Immunology, 2nd ed.; Schat, K.A., Kaspers, B., Kaiser, P., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 121–147. [Google Scholar]
- Porte, R.; Davoudian, S.; Asgari, F.; Parente, R.; Mantovani, A.; Garlanda, C.; Bottazzi, B. The long pentraxin PTX3 as a humoral innate immunity functional player and biomarker of infections and sepsis. Front. Immunol. 2019, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Regulation of innate immune signalling pathways by the tripartite motif (TRIM) family proteins. EMBO Mol. Med. 2011, 3, 513–527. [Google Scholar] [CrossRef] [Green Version]
- Koonpaew, S.; Teeravechyan, S.; Frantz, P.N.; Chailangkarn, T.; Jongkaewwattana, A. PEDV and PDCoV pathogenesis: The interplay between host innate immune responses and porcine enteric coronaviruses. Front. Vet. Sci. 2019, 6, 34. [Google Scholar] [CrossRef]
- Dorrington, M.G.; Fraser, I.D.C. NF-κB signalling in macrophages: Dynamics, crosstalk, and signal integration. Front. Immunol. 2019, 10, 705. [Google Scholar] [CrossRef]
- Abreu, M.T. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat. Rev. Immunol. 2010, 10, 131–144. [Google Scholar] [CrossRef]
- Kaspers, B.; Kaiser, P. Avian antigen-presenting cells. In Avian Immunology, 2nd ed.; Schat, K.A., Kaspers, B., Kaiser, P., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 169–188. [Google Scholar]
- Schokker, D.; Jansman, A.J.M.; Veninga, G.; de Bruin, N.; Vastenhouw, S.A.; de Bree, F.M.; Bossers, A.; Rebel, J.M.J.; Smits, M.A. Perturbation of microbiota in one-day old broiler chickens with antibiotic for 24 hours negatively affects intestinal immune development. BMC Genomics 2017, 18, 241. [Google Scholar] [CrossRef] [PubMed]
- Swaggerty, C.L.; Pevzner, I.Y.; Kogut, M.H. Selection for pro-inflammatory mediators yields chickens with increased resistance against Salmonella enterica serovar Enteritidis. Poult. Sci. 2014, 93, 535–544. [Google Scholar] [CrossRef]
- Genovese, K.J.; He, H.; Swaggerty, C.L.; Kogut, M.H. The avian heterophil. Dev. Comp. Immunol. 2013, 41, 334–340. [Google Scholar] [CrossRef]
- Bar-Shira, E.; Friedman, A. Development and adaptations of innate immunity in the gastrointestinal tract of the newly hatched chick. Dev. Comp. Immunol. 2006, 30, 930–941. [Google Scholar] [CrossRef]
- Basil, M.C.; Levy, B.D. Specialised pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2016, 16, 51. [Google Scholar] [CrossRef]
- Soehnlein, O.; Lindbom, L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 2010, 10, 427–439. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New Adventures of an Old Flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Stewart, D.; Nichol, A. Inflammation, immunity and allergy. Anaesth. Intensive Care Med. 2018, 19, 534–539. [Google Scholar] [CrossRef]
- Headland, S.E.; Norling, L.V. The resolution of inflammation: Principles and challenges. Semin. Immunol. 2015, 27, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Harmon, B.G. Avian heterophils in inflammation and disease resistance. Poult. Sci. 1998, 77, 972–977. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef]
- Quiros, M.; Nusrat, A. Saving problematic mucosae: SPMs in intestinal mucosal inflammation and repair. Trends Mol. Med. 2019, 25, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J. Does promoting resolution instead of inhibiting inflammation represent the new paradigm in treating infections? Mol. Aspects Med. 2017, 58, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Villena, J.; Kitazawa, H. Modulation of intestinal TLR4-inflammatory signaling pathways by probiotic microorganisms: Lessons learned from Lactobacillus jensenii TL2937. Front. Immunol. 2014, 4, 512. [Google Scholar] [CrossRef] [PubMed]
- Voisin, M.B.; Nourshargh, S. Neutrophil trafficking to lymphoid tissues: physiological and pathological implications. J. Pathol. 2019, 247, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Schauer, C.; Janko, C.; Munoz, L.E.; Zhao, Y.; Kienhöfer, D.; Frey, B.; Lell, M.; Manger, B.; Rech, J.; Naschberger, E.; et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 2014, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Maseda, D.; Zackular, J.P.; Trindade, B.; Kirk, L.; Roxas, J.L.; Rogers, L.M.; Washington, M.K.; Du, L.; Koyama, T.; Viswanathan, V.K.; et al. Nonsteroidal anti-inflammatory drugs alter the microbiota and exacerbate Clostridium difficile colitis while dysregulating the inflammatory response. mBio 2019, 10, e02282-18. [Google Scholar] [CrossRef]
- Kogut, M.H.; Tellez, G.I.; McGruder, E.D.; Hargis, B.M.; Williams, J.D.; Corrier, D.E.; DeLoach, J.R. Heterophils are decisive components in the early responses of chickens to Salmonella enteritidis infections. Microb. Pathog. 1994, 16, 141–151. [Google Scholar] [CrossRef]
- Kogut, M.H.; Tellez, G.; Hargis, B.M.; Corrier, D.E.; DeLoach, J.R. The effect of 5-fluorouracil treatment of chicks: A cell depletion model for the study of avian polymorphonuclear leukocytes and natural host defenses. Poult. Sci. 1993, 72, 1873–1880. [Google Scholar] [CrossRef]
- Kogut, M.H.; Lowry, V.K.; Moyes, R.B.; Bowden, L.L.; Bowden, R.; Genovese, K.; Deloach, J.R. Lymphokine-augmented activation of avian heterophils. Poult. Sci. 1998, 77, 964–971. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, M.; Philbin, V.J.; Withanage, G.S.K.; Wigley, P.; Beal, P.K.; Goodchild, M.J.; Barrow, P.; McConnell, I.; Maskell, D.J.; Young, J.; et al. Identification and functional characterization of chicken toll-Like receptor 5 reveals a fundamental role in the biology of infection with Salmonella enterica Serovar Typhimurium. Infect. Immun. 2005, 73, 2344–2350. [Google Scholar] [CrossRef]
- Stegelmeier, A.A.; van Vloten, J.P.; Mould, R.C.; Klafuric, E.M.; Minott, J.A.; Wootton, S.K.; Bridle, B.W.; Karimi, K. Myeloid cells during viral infections and inflammation. Viruses 2019, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Yarovinsky, F. Innate immunity to Toxoplasma gondii infection. Nat. Rev. Immunol. 2014, 14, 109–121. [Google Scholar] [CrossRef]
- Gazendam, R.P.; van de Geer, A.; Roos, D.; van den Berg, T.K.; Kuijpers, T.W. How neutrophils kill fungi. Immunol. Rev. 2016, 273, 299–311. [Google Scholar] [CrossRef]
- Broom, L.J.; Kogut, M.H. Inflammation: friend or foe for animal production? Poult. Sci. 2017, 97, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Broom, L.J.; Kogut, M.H. Deciphering desirable immune responses from disease models with resistant and susceptible chickens. Poult. Sci. 2019, 98, 1634–1642. [Google Scholar] [CrossRef]
- Smith, C.K.; AbuOun, M.; Cawthraw, S.A.; Humphrey, T.J.; Rothwell, L.; Kaiser, P.; Barrow, P.A.; Jones, M.A. Campylobacter colonization of the chicken induces a proinflammatory response in mucosal tissues. FEMS Immunol. Med. Microbiol. 2008, 54, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Foster, N.; Lovell, M.A.; Marston, K.L.; Hulme, S.D.; Frost, A.J.; Bland, P.; Barrow, P.A. Rapid protection of gnotobiotic pigs against experimental salmonellosis following induction of polymorphonuclear leukocytes by avirulent Salmonella enterica. Infect. Immun. 2003, 71, 2182–2191. [Google Scholar] [CrossRef]
- Potey, P.M.; Rossi, A.G.; Lucas, C.D.; Dorward, D.A. Neutrophils in the initiation and resolution of acute pulmonary inflammation: understanding biological function and therapeutic potential. J. Pathol. 2019, 247, 672–685. [Google Scholar] [CrossRef]
- Barrios, M.A.; Da Costa, M.; Kimminau, E.; Fuller, L.; Clark, S.; Pesti, G.; Beckstead, R. Relationship between broiler body weights, Eimeria maxima gross lesion scores, and microscores in three anticoccidial sensitivity tests. Avian Dis. 2017, 61, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Swaggerty, C.L.; Pevzner, I.Y.; Kogut, M.H. Selection for pro-inflammatory mediators produces chickens more resistant to Eimeria tenella. Poult. Sci. 2015, 94, 37–42. [Google Scholar] [CrossRef]
- Ritzi, M.M.; Abdelrahman, W.; Mohnl, M.; Dalloul, R.A. Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poult. Sci. 2014, 93, 2772–2778. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Hu, T.; Rothwell, L.; Vervelde, L.; Kaiser, P.; Boulton, K.; Nolan, M.J.; Tomley, F.M.; Blake, D.P.; Hume, D.A. Analysis of the function of IL-10 in chickens using specific neutralising antibodies and a sensitive capture ELISA. Dev. Comp. Immunol. 2016, 63, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Boulton, K.; Nolan, M.J.; Wu, Z.; Psifidi, A.; Riggio, V.; Harman, K.; Bishop, S.C.; Kaiser, P.; Abrahamsen, M.S.; Hawken, R.; et al. Phenotypic and genetic variation in the response of chickens to Eimeria tenella induced coccidiosis. Genet. Sel. Evol. 2018, 50, 63. [Google Scholar] [CrossRef]
- Villena, J.; Suzuki, R.; Fujie, H.; Chiba, E.; Takahashi, T.; Tomosada, Y.; Shimazu, T.; Aso, H.; Ohwada, S.; Suda, Y.; et al. Immunobiotic Lactobacillus jensenii modulates toll-like receptor 4-induced inflammatory response via negative regulation in porcine antigen presenting cells. Clin. Vaccine Immunol. 2012, 19, 1038–1053. [Google Scholar] [CrossRef]
- Rothwell, L.; Young, J.R.; Zoorob, R.; Whittaker, C.A.; Hesketh, P.; Archer, A.; Smith, A.L.; Kaiser, P. Cloning and characterization of chicken IL-10 and its role in the immune response to Eimeria maxima. J. Immunol. 2004, 173, 2675–2682. [Google Scholar] [CrossRef] [PubMed]
- Arendt, M.K.; Sand, J.M.; Marcone, T.M.; Cook, M.E. Interleukin-10 neutralizing antibody for detection of intestinal luminal levels and as a dietary additive in Eimeria challenged broiler chicks. Poult. Sci. 2016, 95, 430–438. [Google Scholar] [CrossRef]
- Sand, J.M.; Arendt, M.K.; Repasy, A.; Deniz, G.; Cook, M.E. Oral antibody to interleukin-10 reduces growth rate depression due to Eimeria spp. infection in broiler chickens. Poult. Sci. 2016, 95, 439–446. [Google Scholar]
- Rutigliano, J.A.; Graham, B.S. Prolonged production of TNF-alpha exacerbates illness during respiratory syncytial virus infection. J. Immunol. 2004, 173, 3408–3417. [Google Scholar] [CrossRef] [PubMed]
- Irvine, K.M.; Ratnasekera, I.; Powell, E.E.; Hume, D.A. Causes and consequences of innate immune dysfunction in cirrhosis. Front. Immunol. 2019, 10, 293. [Google Scholar] [CrossRef]
- Aricibasi, M.; Jung, A.; Heller, E.D.; Rautenschlein, S. Differences in genetic background influence the induction of innate and acquired immune responses in chickens depending on the virulence of the infecting infectious bursal disease virus (IBDV) strain. Vet. Immunol. Immunopathol. 2010, 135, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Berndt, A.; Methner, U. B cell and macrophage response in chicks after oral administration of Salmonella typhimurium strains. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 235–246. [Google Scholar] [CrossRef]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef]
- Minozzi, S.; Bonovas, S.; Lytras, T.; Pecoraro, V.; González-Lorenzo, M.; Bastiampillai, A.J.; Gabrielli, E.M.; Lonati, A.C.; Moja, L.; Cinquini, M.; et al. Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin. Drug Saf. 2016, 15 (Suppl. 1), 11–34. [Google Scholar] [CrossRef]
- Izcue, A.; Coombes, J.L.; Powrie, F. Regulatory lymphocytes and intestinal inflammation. Annu. Rev. Immunol. 2009, 27, 313–338. [Google Scholar] [CrossRef]
- Neurath, M.F. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 269–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kogut, M.H.; Genovese, K.J.; Swaggerty, C.L.; He, H.; Broom, L. Inflammatory phenotypes in the intestine of poultry: not all inflammation is created equal. Poult. Sci. 2018, 97, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- Wentowski, C.; Mewada, N.; Nielsen, N.D. Sepsis in 2018: A review. Anaesth. Intensive Care Med. 2019, 20, 6–13. [Google Scholar] [CrossRef]
- Kumar, V. Immunometabolism: another road to sepsis and its therapeutic targeting. Inflammation 2018. [Google Scholar] [CrossRef]
- Raymond, S.L.; Hawkins, R.B.; Stortz, J.A.; Murphy, T.J.; Ungaro, R.; Dirain, M.L.; Nacionales, D.C.; Hollen, M.K.; Rincon, J.C.; Larson, S.D.; et al. Sepsis is associated with reduced spontaneous neutrophil migration velocity in human adults. PLoS ONE 2018, 13, e0205327. [Google Scholar] [CrossRef] [PubMed]
- Schulte, W.; Bernhagen, J.; Bucala, R. Cytokines in sepsis: Potent immunoregulators and potential therapeutic targets—An updated view. Mediators Inflamm. 2013, 2013, 165974. [Google Scholar] [CrossRef]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.W.; Pires, E.; et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity 2019, 50, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut–lung axis. Mucosal Immunol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, R.J.; Kogut, M.H. Immunometabolism and the kinome peptide array: A new perspective and tool for the study of gut health. Front. Vet. Sci. 2015, 2, 44. [Google Scholar] [CrossRef]
- Ashley, N.T.; Weil, Z.M.; Nelson, R.J. Inflammation: Mechanisms, costs, and natural variation. Annu. Rev. Ecol. Syst. 2012, 43, 385–406. [Google Scholar] [CrossRef]
- Garrido, D.; Chanteloup, N.K.; Trotereau, A.; Lion, A.; Bailleul, G.; Esnault, E.; Trapp, S.; Quéré, P.; Schouler, C.; Guabiraba, R. Characterization of the phospholipid platelet-activating factor as a mediator of inflammation in chickens. Front. Vet. Sci. 2017, 4, 226. [Google Scholar] [CrossRef] [PubMed]
- Jeffers, T.K.; Wagenbach, G.E. Sex differences in embryonic response to Eimeria tenella infection. J. Parasitol. 1969, 55, 949–951. [Google Scholar] [CrossRef]
- Cox, C.M.; Sumners, L.H.; Kim, S.; McElroy, A.P.; Bedford, M.R.; Dalloul, R.A. Immune responses to dietary beta-glucan in broiler chicks during an Eimeria challenge. Poult. Sci. 2010, 89, 2597–2607. [Google Scholar] [CrossRef]
- St. Paul, M.; Brisbin, J.T.; Abdul-Careem, M.F.; Sharif, S. Immunostimulatory properties of Toll-like receptor ligands in chickens. Vet. Immunol. Immunopathol. 2013, 152, 191–199. [Google Scholar] [CrossRef] [PubMed]
| Polyunsaturated Fatty Acid Precursors | Pro-Resolving Family/Series |
|---|---|
| Arachidonic acid (C20:4) | Lipoxin |
| Eicosapentanoic acid (C20:5) | Resolvin E |
| Docosapentaenoic acid (C22:5) | Resolvin D, E & T, Maresin, Protectin |
| Docosahexanoic acid (C22:6) | Resolvin D, Maresin, Protectin |
| Strategy | Example(s) | Desired Outcome |
|---|---|---|
| Breeding | ||
| ↑ Resistance | Resist infection (and/or) | |
| ↑ Tolerance | Tolerate consequences (and/or) | |
| ↑ Resilience | Recover promptly | |
| Exogenous | ||
| Prime innate immune cells | PRR ligands | Potentiate immune cells |
| Immunometabolism | SCFA | Influence immune cell phenotype & function |
| Resolution | ω-3 and ω-6 essential fatty acids | Promote production of pro-resolving mediators |
| Microbiome-host interactions | Probiotics Prebiotics Phytogenics Organic acids Enzymes | Influence gut microbiome function and/or host responses |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broom, L.J. Host–Microbe Interactions and Gut Health in Poultry—Focus on Innate Responses. Microorganisms 2019, 7, 139. https://doi.org/10.3390/microorganisms7050139
Broom LJ. Host–Microbe Interactions and Gut Health in Poultry—Focus on Innate Responses. Microorganisms. 2019; 7(5):139. https://doi.org/10.3390/microorganisms7050139
Chicago/Turabian StyleBroom, Leon J. 2019. "Host–Microbe Interactions and Gut Health in Poultry—Focus on Innate Responses" Microorganisms 7, no. 5: 139. https://doi.org/10.3390/microorganisms7050139
APA StyleBroom, L. J. (2019). Host–Microbe Interactions and Gut Health in Poultry—Focus on Innate Responses. Microorganisms, 7(5), 139. https://doi.org/10.3390/microorganisms7050139