Relevant Aspects of Clostridium estertheticum as a Specific Spoilage Organism of Vacuum-Packed Meat
Abstract
:1. Introduction
2. Taxonomic Classification of Clostridium estertheticum
3. Isolation and Conventional Culturing of Clostridium estertheticum
4. Molecular and Non-Molecular Based Identification of Clostridium estertheticum
5. Growth and Metabolism of Clostridium estertheticum
6. Clostridium estertheticum as a Causative Agent of Blown Pack Spoilage
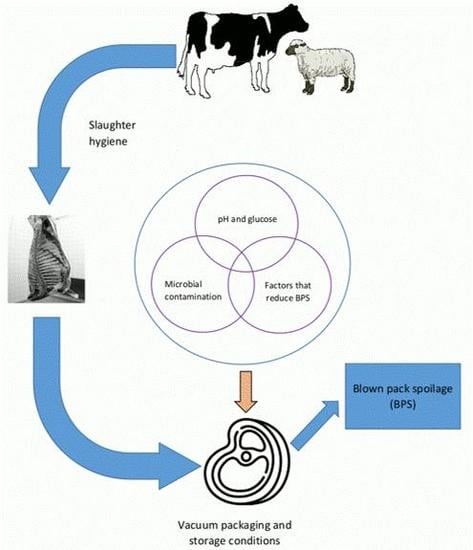
7. Factors Affecting Blown Pack Spoilage by Clostridium estertheticum
8. Intervention and Inactivation Strategies to Reduce Blown Pack Spoilage by Clostridium estertheticum
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hilgarth, M.; Behr, J.; Vogel, R.F. Monitoring of spoilage-associated microbiota on modified atmosphere packaged beef and differentiation of psychrophilic and psychrotrophic strains. J. Appl. Microbiol. 2018, 124, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Höll, L.; Behr, J.; Vogel, R.F. Identification and growth dynamics of meat spoilage microorganisms in modified atmosphere packaged poultry meat by MALDI-TOF MS. Food Microbiol. 2016, 60, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Lahmar, A.; Morcuende, D.; Andrade, M.-J.; Chekir-Ghedira, L.; Estévez, M. Prolonging shelf life of lamb cutlets packed under high-oxygen modified atmosphere by spraying essential oils from North-African plants. Meat Sci. 2018, 139, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Mikš-Krajnik, M.; Yoon, Y.-J.; Ukuku, D.O.; Yuk, H.-G. Volatile chemical spoilage indexes of raw atlantic salmon (Salmo salar) stored under aerobic condition in relation to microbiological and sensory shelf lives. Food Microbiol. 2016, 53, 182–191. [Google Scholar] [CrossRef]
- Cheng, W.; Sun, D.-W.; Pu, H.; Wei, Q. Chemical spoilage extent traceability of two kinds of processed pork meats using one multispectral system developed by hyperspectral imaging combined with effective variable selection methods. Food Chem. 2017, 221, 1989–1996. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Kerry, J.P.; Hopkins, D.L. Meat packaging solutions to current industry challenges: A review. Meat Sci. 2018, 144, 159–168. [Google Scholar] [CrossRef]
- Nychas, G.-J.E.; Skandamis, P.N.; Tassou, C.C.; Koutsoumanis, K.P. Meat spoilage during distribution. Meat Sci. 2008, 78, 77–89. [Google Scholar] [CrossRef]
- Stellato, G.; La Storia, A.; De Filippis, F.; Borriello, G.; Villani, F.; Ercolini, D. Overlap of spoilage-associated microbiota between meat and the meat processing environment in small-scale and large-scale retail distributions. Appl. Environ. Microbiol. 2016, 82, 4045–4054. [Google Scholar] [CrossRef]
- Wambui, J.; Lamuka, P.; Karuri, E.; Matofari, J.; Njage, P.M.K. Microbial contamination level profiles attributed to contamination of beef carcasses, personnel, and equipment: Case of small and medium enterprise slaughterhouses. J. Food Prot. 2018, 81, 684–691. [Google Scholar] [CrossRef]
- Fletcher, B.; Mullane, K.; Platts, P.; Todd, E.; Power, A.; Roberts, J.; Chapman, J.; Cozzolino, D.; Chandra, S. Advances in meat spoilage detection: A short focus on rapid methods and technologies. CyTA J. Food 2018, 16, 1037–1044. [Google Scholar] [CrossRef]
- Hilgarth, M.; Nani, M.; Vogel, R.F. Assertiveness of meat-borne Lactococcus piscium strains and their potential for competitive exclusion of spoilage bacteria in situ and in vitro. J. Appl. Microbiol. 2018, 124, 1243–1253. [Google Scholar] [CrossRef]
- Gram, L.; Ravn, L.; Rasch, M.; Bruhn, J.B.; Christensen, A.B.; Givskov, M. Food spoilage-interactions between food spoilage bacteria. Int. J. Food Microbiol. 2002, 78, 79–97. [Google Scholar] [CrossRef]
- Andreevskaya, M.; Jääskeläinen, E.; Johansson, P.; Ylinen, A.; Paulin, L.; Björkroth, J.; Auvinen, P. Food spoilage-associated Leuconostoc, Lactococcus, and Lactobacillus species display different survival strategies in response to competition. Appl. Environ. Microbiol. 2018, 84, e00554-18. [Google Scholar] [CrossRef] [PubMed]
- Casaburi, A.; Piombino, P.; Nychas, G.-J.; Villani, F.; Ercolini, D. Bacterial populations and the volatilome associated to meat spoilage. Food Microbiol. 2015, 45, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Van Rooyen, L.A.; Allen, P.; Kelly-Rees, C.; O’Connor, D.I. The Effects of varying gas concentrations and exposure times on colour stability and shelf-life of vacuum packaged beef steaks subjected to carbon monoxide pretreatment. Food Packag. Shelf Life 2018, 18, 230–237. [Google Scholar] [CrossRef]
- De Filippis, F.; La Storia, A.; Villani, F.; Ercolini, D. Strain-level diversity analysis of Pseudomonas fragi after in situ pangenome reconstruction shows distinctive spoilage-associated metabolic traits clearly selected by different storage conditions. Appl. Environ. Microbiol. 2019, 85, e02212-18. [Google Scholar] [CrossRef] [PubMed]
- Lavieri, N.; Williams, S.K. Effects of packaging systems and fat concentrations on microbiology, sensory and physical properties of ground beef stored at 4 ± 1 °C for 25 days. Meat Sci. 2014, 97, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Mansur, A.R.; Song, E.-J.; Cho, Y.-S.; Nam, Y.-D.; Choi, Y.-S.; Kim, D.-O.; Seo, D.-H.; Nam, T.G. Comparative evaluation of spoilage-related bacterial diversity and metabolite profiles in chilled beef stored under air and vacuum packaging. Food Microbiol. 2019, 77, 166–172. [Google Scholar] [CrossRef]
- Doulgeraki, A.I.; Ercolini, D.; Villani, F.; Nychas, G.-J.E. Spoilage microbiota associated to the storage of raw meat in different conditions. Int. J. Food Microbiol. 2012, 157, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.; Yang, X.; Hopkins, D.L.; Zhu, L.; Dong, P.; Liang, R.; Luo, X. Shelf-life and microbial community dynamics of super-chilled beef imported from Australia to China. Food Res. Int. 2019, 120, 784–792. [Google Scholar] [CrossRef]
- Pothakos, V.; Devlieghere, F.; Villani, F.; Björkroth, J.; Ercolini, D. Lactic Acid Bacteria and their controversial role in fresh meat spoilage. Meat Sci. 2015, 109, 66–74. [Google Scholar] [CrossRef]
- Zhang, P.; Badoni, M.; Gänzle, M.; Yang, X. Growth of Carnobacterium spp. isolated from chilled vacuum-packaged meat under relevant acidic conditions. Int. J. Food Microbiol. 2018, 286, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Chaillou, S.; Chaulot-Talmon, A.; Caekebeke, H.; Cardinal, M.; Christieans, S.; Denis, C.; Hélène Desmonts, M.; Dousset, X.; Feurer, C.; Hamon, E.; et al. Origin and ecological selection of core and food-specific bacterial communities associated with meat and seafood spoilage. ISME J. 2015, 9, 1105–1118. [Google Scholar] [CrossRef]
- Hultman, J.; Rahkila, R.; Ali, J.; Rousu, J.; Björkroth, K.J. Meat processing plant microbiome and contamination patterns of cold-tolerant bacteria causing food safety and spoilage risks in the manufacture of vacuum-packaged cooked sausages. Appl. Environ. Microbiol. 2015, 81, 7088–7097. [Google Scholar] [CrossRef] [PubMed]
- Dorn-In, S.; Schwaiger, K.; Springer, C.; Barta, L.; Ulrich, S.; Gareis, M. Development of a Multiplex QPCR for the species identification of Clostridium estertheticum, C. frigoriphilum, C. bowmanii and C. tagluense-like from blown pack spoilage (BPS) meats and from wild boars. Int. J. Food Microbiol. 2018, 286, 162–169. [Google Scholar] [CrossRef] [PubMed]
- André, S.; Vallaeys, T.; Planchon, S. Spore-forming bacteria responsible for food spoilage. Res. Microbiol. 2017, 168, 379–387. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, J.; Yuan, Y.; Yue, T. Diversity and characterization of spoilage-associated psychrotrophs in food in cold chain. Int. J. Food Microbiol. 2019, 290, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Brightwell, G.; Clemens, R. Development and validation of a Real-Time PCR assay specific for Clostridium estertheticum and C. estertheticum-like psychrotolerant bacteria. Meat Sci. 2012, 92, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.D.; Lawson, P.A.; Willems, A.; Cordoba, J.J.; Fernandez-Garayzabal, J.; Garcia, P.; Cai, J.; Hippe, H.; Farrow, J.A.E. The phylogeny of the genus Clostridium: Proposal of five new genera and eleven new species combinations. Int. J. Syst. Bacteriol. 1994, 44, 812–826. [Google Scholar] [CrossRef] [PubMed]
- Vos, P.; Garrity, G.M.; Jones, D.; Krieg, N.R.; Ludwig, W.; Rainey, F.A.; Schleifer, K.-H. Bergey’s Manual of Systematic Bacteriology, Volume 3: The Firmicutes; Springer: Berlin/Heidelberg, Germany, 2012; p. 1450. [Google Scholar]
- Moschonas, G.; Bolton, D.J.; Sheridan, J.J.; McDowell, D.A. The effect of storage temperature and inoculum level on the time of onset of “blown pack” spoilage. J. Appl. Microbiol. 2010, 108, 532–539. [Google Scholar] [CrossRef]
- Dainty, R.H.; Edwards, R.A.; Hibbard, C.M. Spoilage of vacuum-packed beef by Aclostridium sp. J. Sci. Food Agric. 1989, 49, 473–486. [Google Scholar] [CrossRef]
- Kalchayanand, N.; Ray, B.; Field, R.A.; Johnson, M.C. Spoilage of vacuum-packaged refrigerated beef by Clostridium. J. Food Prot. 1989, 52, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Broda, D.M.; Musgrave, D.R.; Bell, R.G. Use of Restriction Fragment Length Polymorphism analysis to differentiate strains of psychrophilic and psychrotrophic Clostridia associated with “blown pack” spoilage of vacuum-packed meats. J. Appl. Microbiol. 2000, 88, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Byrne, B.; Monaghan, A.M.; Lyng, J.G.; Sheridan, J.J.; Bolton, D.J. A case of “blown pack” meat linked to Clostridium estertheticum in Ireland. J. Food Saf. 2009, 29, 629–635. [Google Scholar] [CrossRef]
- Joseph, R.C.; Kim, N.M.; Sandoval, N.R. Recent developments of the synthetic biology toolkit for Clostridium. Front. Microbiol. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Prazmowski, A. Untersuchung über die Entwickelungsgeschichte und Fermentwirking einiger Bakterien-arten. Inaugural Dissertation, Hugo Voigt, Leipzig, Germany, 1880. [Google Scholar]
- Alou, M.T.; Ndongo, S.; Frégère, L.; Labas, N.; Andrieu, C.; Richez, M.; Couderc, C.; Baudoin, J.P.; Abrahão, J.; Brah, S.; et al. Taxonogenomic description of four new Clostridium species isolated from human gut: ‘Clostridium amazonitimonense’, ‘Clostridium merdae’, ‘Clostridium massilidielmoense’ and ‘Clostridium nigeriense’. New Microbes New Infect. 2018, 21, 128–139. [Google Scholar] [CrossRef]
- Lawson, P.A.; Rainey, F.A. Proposal to restrict the Genus Clostridium prazmowski to Clostridium butyricum and related species. Int. J. Syst. Evol. Microbiol. 2016, 66, 1009–1016. [Google Scholar] [CrossRef]
- Oren, A.; Rupnik, M. Clostridium difficile and Clostridioides difficile: Two validly published and correct names. Anaerobe 2018, 52, 125–126. [Google Scholar] [CrossRef]
- Collins, M.D. Taxonomic studies on a psychrophilic Clostridium from vacuum-packed beef: Description of Clostridium estertheticum sp. nov. FEMS Microbiol. Lett. 1992, 96, 235–239. [Google Scholar] [CrossRef]
- Kalchayanand, N.; Ray, B.; Field, R.A. Characteristics of psychrotrophic Clostridium laramie causing spoilage of vacuum-packaged refrigerated fresh and roasted beef. J. Food Prot. 1993, 56, 13–17. [Google Scholar] [CrossRef]
- Spring, S. Characterization of novel psychrophilic Clostridia from an antarctic microbial mat: Description of Clostridium frigoris sp. nov., Clostridium lacusfryxellense sp. nov., Clostridium bowmanii sp. nov. and Clostridium psychrophilum sp. nov. and reclassification of Clostridium laramiense as Clostridium estertheticum subsp. laramiense subsp. nov. Int. J. Syst. Evol. Microbiol. 2003, 53, 1019–1029. [Google Scholar]
- Yu, Z.; Gunn, L.; Brennan, E.; Reid, R.; Wall, P.G.; Gaora, P.; Hurley, D.; Bolton, D.; Fanning, S. Complete genome sequence of Clostridium estertheticum DSM 8809, a microbe identified in spoiled vacuum-packed beef. Front. Microbiol. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Yang, X.; Gill, C.O.; Balamurugan, S. Products of glucose and lactate fermentation, and utilization of amino acids by Clostridium estertheticum ssp. laramiense and estertheticum growing in meat juice medium. J. Food Prot. 2016, 73, 1348–1352. [Google Scholar]
- Broda, D.M.; Delacy, K.M.; Bell, R.G.; Braggins, T.J.; Cook, R.L. Psychrotrophic Clostridium spp. associated with ‘blown pack’ spoilage of chilled vacuum-packed red meats and dog rolls in gas-impermeable plastic casings. Int. J. Food Microbiol. 1996, 29, 335–352. [Google Scholar] [CrossRef]
- Boerema, J.A.; Broda, D.M.; Bell, R.G. Abattoir sources of psychrophilic Clostridia causing blown pack spoilage of vacuum-packed chilled meats determined by culture-based and molecular detection procedures. Lett. Appl. Microbiol. 2003, 36, 406–411. [Google Scholar] [CrossRef]
- Moschonas, G.; Bolton, D.J.; Sheridan, J.J.; McDowell, D.A. Isolation and sources of blown pack spoilage Clostridia in beef abattoirs. J. Appl. Microbiol. 2009, 107, 616–624. [Google Scholar] [CrossRef]
- Broda, D.M. The Effect of peroxyacetic acid-based sanitizer, heat and ultrasonic waves on the survival of Clostridium estertheticum spores in vitro. Lett. Appl. Microbiol. 2007, 45, 336–341. [Google Scholar] [CrossRef]
- Bonke, R.; Drees, N.; Gareis, M. Detection of psychrophilic and psychrotolerant Clostridium spp. in chilled fresh vacuum-packed meat using different PCR methods. FEMS Microbiol. Lett. 2015, 363, fnv218. [Google Scholar] [CrossRef]
- Jones, R.J.; Zagorec, M.; Brightwell, G.; Tagg, J.R. Inhibition by Lactobacillus sakei of other species in the flora of vacuum packaged raw meats during prolonged storage. Food Microbiol. 2009, 26, 876–881. [Google Scholar] [CrossRef]
- Moschonas, G.; Bolton, D.J.; McDowell, D.A.; Sheridan, J.J. Diversity of culturable psychrophilic and psychrotrophic anaerobic bacteria isolated from beef abattoirs and their environments. Appl. Environ. Microbiol. 2011, 77, 4280–4284. [Google Scholar] [CrossRef]
- Bakhtiary, F.; Sayevand, H.R.; Remely, M.; Hippe, B.; Indra, A.; Hosseini, H.; Haslberger, A.G. Identification of Clostridium spp. derived from a sheep and cattle slaughterhouse by Matrix-Assisted Laser Desorption and Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS) and 16S RDNA sequencing. J. Food Sci. Technol. 2018, 55, 3232–3240. [Google Scholar] [CrossRef]
- Yang, X.; Gill, C.O.; Balamurugan, S. Enumeration of Clostridium estertheticum spores in samples from meat plant conveyors and silage stacks by conventional and Real-Time PCR procedures. Internet J. Food Saf. 2010, 12, 115–121. [Google Scholar]
- Helps, C.R.; Harbour, D.A.; Corry, J.E.L. PCR-based 16S ribosomal DNA detection technique for Clostridium estertheticum causing spoilage in vacuum-packed chill-stored beef. Int. J. Food Microbiol. 1999, 52, 57–65. [Google Scholar] [CrossRef]
- Broda, D.M.; Bell, R.G.; Boerema, J.A.; Musgrave, D.R. The abattoir source of culturable psychrophilic Clostridium spp. causing “blown pack” spoilage of vacuum-packed chilled venison. J. Appl. Microbiol. 2002, 93, 817–824. [Google Scholar] [CrossRef]
- Broda, D.M.; Boerema, J.A.; Bell, R.G. PCR Detection of psychrophilic Clostridium spp. causing “blown pack” spoilage of vacuum-packed chilled meats. J. Appl. Microbiol. 2003, 94, 515–522. [Google Scholar] [CrossRef]
- Boerema, J.A.; Broda, D.M.; Penney, N.; Brightwell, G. Influence of peroxyacetic acid–based carcass rinse on the onset of “blown pack” spoilage in artificially inoculated vacuum-packed chilled beef. J. Food Prot. 2007, 70, 1434–1439. [Google Scholar] [CrossRef]
- Brightwell, G.; Broda, D.M.; Boerema, J.A. Sources of psychrophilic and psychrotolerant Clostridia causing spoilage of vacuum-packed chilled meats, as determined by PCR amplification procedure. J. Appl. Microbiol. 2009, 107, 178–186. [Google Scholar]
- Reid, R.; Fanning, S.; Whyte, P.; Kerry, J.; Bolton, D. Comparison of hot versus cold boning of beef carcasses on bacterial growth and the risk of blown pack spoilage. Meat Sci. 2017, 125, 46–52. [Google Scholar] [CrossRef]
- Brightwell, G.; Horváth, K.M. Molecular discrimination of New Zealand sourced meat spoilage associated psychrotolerant Clostridium species by ARDRA and its comparison with 16s RNA gene sequencing. Meat Sci. 2018, 138, 23–27. [Google Scholar] [CrossRef]
- Rajagopal, S.; McMullen, L.M.; Gill, C.O.; Yang, X. Characterization of germination of spores of Clostridium estertheticum, the primary causative agent of blown pack spoilage of vacuum packaged beef. Food Res. Int. 2016, 87, 109–114. [Google Scholar] [CrossRef]
- Yang, X.; Gill, C.O.; Balamurugan, S. Effects of temperature and pH on the growth of bacteria isolated from blown packs of vacuum-packaged beef. J. Food Prot. 2009, 72, 2380–2385. [Google Scholar] [CrossRef]
- Clemens, R.M.; Adam, K.H.; Brightwell, G. Contamination levels of Clostridium estertheticum spores that result in gaseous spoilage of vacuum-packaged chilled beef and lamb meat. Lett. Appl. Microbiol. 2010, 50, 591–596. [Google Scholar] [CrossRef]
- Yang, X.; Balamurugan, S.; Gill, C.O. Substrate utilization by Clostridium estertheticum cultivated in meat juice medium. Int. J. Food Microbiol. 2009, 128, 501–505. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Badoni, M. Effects of meat pH and the initial numbers of spores of Clostridium estertheticum on the development of blown pack spoilage of vacuum-packaged beef. Int. J. Food Sci. Technol. 2014, 49, 1619–1625. [Google Scholar] [CrossRef]
- Brightwell, G.; Clemens, R.; Urlich, S.; Boerema, J. Possible involvement of psychrotolerant Enterobacteriaceae in blown pack spoilage of vacuum-packaged raw meats. Int. J. Food Microbiol. 2007, 119, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-H.; Arthur, T.M.; Siragusa, G.R. Gas formation in ground beef chubs due to Hafnia alvei is reduced by multiple applications of antimicrobial interventions to artificially inoculated beef trim stock. J. Food Prot. 2002, 65, 1651–1655. [Google Scholar] [CrossRef]
- Chaves, R.D.; Silva, A.R.; Sant’Ana, A.S.; Campana, F.B.; Massaguer, P.R. Gas-producing and spoilage potential of Enterobacteriaceae and Lactic Acid Bacteria isolated from chilled vacuum-packaged beef. Int. J. Food Sci. Technol. 2012, 47, 1750–1756. [Google Scholar] [CrossRef]
- Bolton, D.J.; Carroll, J.; Walsh, D. A four-year survey of blown pack spoilage Clostridium estertheticum and Clostridium gasigenes on beef primal cuts. Lett. Appl. Microbiol. 2015, 61, 153–157. [Google Scholar] [CrossRef]
- Yang, X.; Youssef, M.K.; Gill, C.O.; Badoni, M.; López-Campos, Ó. Effects of meat pH on growth of 11 species of psychrotolerant Clostridia on vacuum packaged beef and blown pack spoilage of the product. Food Microbiol. 2014, 39, 13–18. [Google Scholar] [CrossRef]
- Silva, A.R.; Tahara, A.C.C.; Chaves, R.D.; Sant’Ana, A.S.; Faria, J.d.A.F.; Massaguer, P.R. Influence of different shrinking temperatures and vacuum conditions on the ability of psychrotrophic Clostridium to cause “blown pack” spoilage in chilled vacuum-packaged beef. Meat Sci. 2012, 92, 498–505. [Google Scholar] [CrossRef]
- Jones, R.J.; Hussein, H.M.; Zagorec, M.; Brightwell, G.; Tagg, J.R. Isolation of Lactic Acid Bacteria with inhibitory activity against pathogens and spoilage organisms associated with fresh meat. Food Microbiol. 2008, 25, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.; Carvalho, J.; Massaguer, P.R. “Blown pack” probabilistic modeling for C. algidicarnis and C. estertheticum under the effects of storage temperature, vacuum level and package shrink temperature. Procedia Food Sci. 2016, 7, 59–62. [Google Scholar] [CrossRef]
- Reid, R.; Fanning, S.; Whyte, P.; Kerry, J.; Bolton, D. An investigation of the effect of rapid slurry chilling on blown pack spoilage of vacuum-packaged beef primals. Lett. Appl. Microbiol. 2017, 64, 177–181. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Balamurugan, S.; Gill, C.O. Effects on the development of blown pack spoilage of the initial numbers of Clostridium estertheticum spores and Leuconostoc mesenteroides on vacuum packed beef. Meat Sci. 2011, 88, 361–367. [Google Scholar] [CrossRef]
- Mills, J.; Donnison, A.; Brightwell, G. Factors affecting microbial spoilage and shelf-life of chilled vacuum-packed lamb transported to distant markets: A review. Meat Sci. 2014, 98, 71–80. [Google Scholar] [CrossRef]
- James, S.J.; James, C. Meat Refrigeration; CRC Press: Boca Raton, FL, USA, 2002; p. 360. [Google Scholar]
- Moschonas, G.; Bolton, D.J.; Sheridan, J.J.; McDowell, D.A. The effect of heat shrink treatment and storage temperature on the time of onset of “blown pack” spoilage. Meat Sci. 2011, 87, 115–118. [Google Scholar] [CrossRef]
- Bell, R.G.; Moorhead, S.M.; Broda, D.M. Influence of heat shrink treatments on the onset of Clostridial “blown pack” spoilage of vacuum-packed chilled meat. Food Res. Int. 2001, 34, 271–275. [Google Scholar] [CrossRef]
- Sumner, J.; Jenson, I.; Ross, T. Using predictive microbiology to benefit the Australian meat industry. In Case Studies in Food Safety and Authenticity; Elsevier: Amsterdam, The Netherlands, 2012; pp. 276–283. [Google Scholar]
- Claus, J.R.; Sørheim, O. Preserving pre-rigor meat functionality for beef patty production. Meat Sci. 2006, 73, 287–294. [Google Scholar] [CrossRef]
- Sukumaran, A.T.; Holtcamp, A.J.; Campbell, Y.L.; Burnett, D.; Schilling, M.W.; Dinh, T.T.N. Technological characteristics of pre- and post-rigor deboned beef mixtures from Holstein steers and quality attributes of cooked beef sausage. Meat Sci. 2018, 145, 71–78. [Google Scholar] [CrossRef]
- Mills, J.; Horváth, K.M.; Brightwell, G. Antimicrobial effect of different peroxyacetic acid and hydrogen peroxide formats against spores of Clostridium estertheticum. Meat Sci. 2018, 143, 69–73. [Google Scholar] [CrossRef]
- Reid, R.; Bolton, D.; Tiuftin, A.; Kerry, J.; Fanning, S.; Whyte, P. Controlling blown pack spoilage using anti-microbial packaging. Foods 2017, 6, 67. [Google Scholar] [CrossRef] [PubMed]
| Substance | g/L or mL/L |
|---|---|
| Proteose Peptone | 5 |
| Tryptone | 5 |
| Yeast extract | 10 |
| Meat extract Powder | 10 |
| Glucose | 2 |
| Soluble starch | 1 |
| Resazurin | 0.001 |
| Cysteine HCl | 0.2 |
| Solution of Silicon Antifoaming Agent 20% | 0.25 |
| Salts Solution A | 40 |
| Salts Solution B | 40 |
| Salts Solution A | |
| CaCl2·2H2O | 0.265 |
| MgSO4·7H2O | 0.48 |
| NaCl | 2 |
| Salts Solution B | |
| KH2PO4 | 1 |
| K2HPO4·3H2O | 1.3 |
| NaHCO3 | 10 |
| Assay | Primer and Probe | Sequence | Reference | |
|---|---|---|---|---|
| PCR | Primer | 16SEF | 5′-TCG GAA TTT CAC TTT GAG-3′ | [57] |
| 16SER | 5′-AAG GAC TTC ACT CAT CTC TG-3′ | |||
| RT-PCR | Primer | TMF | 5′-CGG CGG ACG GGT GAG TAA C-3′ | [28] |
| TMR | 5′-CGG GTC CAT CTC AAA GTG RAA CT-3′ | |||
| Probe | 5′-FAM-CGT GGG TAA CCT GCC TCA AAG AGG GG-TAMRA-3′ | |||
| qPCR | Primer | TMF | 5′-CGGCGGACGGGTGAGTAAC-3′ | [25] |
| Cl642-R | 5′-CCTCTCCTGCACTCTAGA-3′ | |||
| Probe | Cest | 5′-HEX-CAAAGGAATTTTTCGGAATTTCACTTTGAG-BHQ1-3′ | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wambui, J.; Stephan, R. Relevant Aspects of Clostridium estertheticum as a Specific Spoilage Organism of Vacuum-Packed Meat. Microorganisms 2019, 7, 142. https://doi.org/10.3390/microorganisms7050142
Wambui J, Stephan R. Relevant Aspects of Clostridium estertheticum as a Specific Spoilage Organism of Vacuum-Packed Meat. Microorganisms. 2019; 7(5):142. https://doi.org/10.3390/microorganisms7050142
Chicago/Turabian StyleWambui, Joseph, and Roger Stephan. 2019. "Relevant Aspects of Clostridium estertheticum as a Specific Spoilage Organism of Vacuum-Packed Meat" Microorganisms 7, no. 5: 142. https://doi.org/10.3390/microorganisms7050142
APA StyleWambui, J., & Stephan, R. (2019). Relevant Aspects of Clostridium estertheticum as a Specific Spoilage Organism of Vacuum-Packed Meat. Microorganisms, 7(5), 142. https://doi.org/10.3390/microorganisms7050142