Combined Effect of Naturally-Derived Biofilm Inhibitors and Differentiated HL-60 Cells in the Prevention of Staphylococcus aureus Biofilm Formation
Abstract
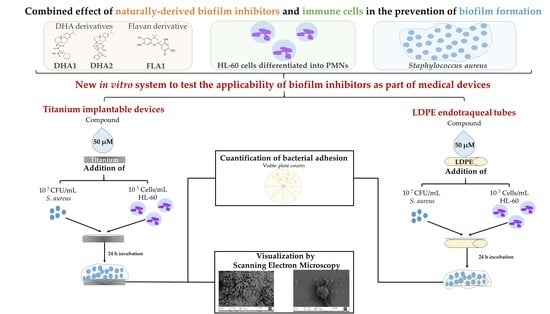
:1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. Bacterial Strains
2.3. HL-60 Cell Culture and Differentiation
2.4. Biofilm Prevention Efficacy of Differentiated HL-60 Cells against Different Bacterial Concentrations of S. aureus ATCC 25923
2.4.1. Bacterial Inoculum Preparation
2.4.2. Immune Cell (HL-60) Preparation
2.4.3. Coculture of S. aureus ATCC 25923 and HL-60 Cells
2.4.4. Biofilm Quantification in 96-Well Microplates
2.5. Influence of Opsonizing S. aureus ATCC 25923 on the Efficacy of HL-60 Cells in Preventing Bacterial Attachment on Titanium Coupons
2.6. Effect of the Antimicrobial Compounds on the Prevention of S. aureus ATCC 25923 and S. aureus P2 Adhesion in Coculture with Differentiated HL-60 Cells on Titanium Coupons
2.6.1. Culture of Staphylococci and HL-60 Cells
2.6.2. Bacterial Adherence on Titanium Coupons
2.7. Effect of the Antimicrobial Compounds on the Prevention of S. aureus ATCC 25923 Adhesion in Coculture with Differentiated HL-60 Cells on LDPE Tubes
2.8. Scanning Electron Microscopy (SEM)
2.9. Statistical Analysis
3. Results
3.1. Effect of PMA Activation of Differentiated HL-60 Cells on Prevention of S. aureus ATCC 25923 Biofilm Formation
3.2. Influence of Opsonizing S. aureus ATCC 25923 on the Efficacy of HL-60 Cells in Preventing Bacterial Attachment on Titanium Coupons
3.3. Effect of the Antimicrobial Compounds on the Prevention of S. aureus Adhesion in Coculture with Differentiated HL-60 Cells on Titanium Coupons
3.4. Effect of the Antimicrobial Compounds on the Prevention of S. aureus ATCC 25923 Adhesion in Coculture with Differentiated HL-60 Cells on LDPE tubes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Friedrich, A.W. Control of hospital acquired infections and antimicrobial resistance in Europe: The way to go. Wien. Med. Wochenschr. 2019, 169, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, H.A.; Baig, F.K.; Mehboob, R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pac. J. Trop. Biomed. 2017, 7, 478–482. [Google Scholar] [CrossRef]
- Paharik, A.E.; Horswill, A.R. The Staphylococcal Biofilm: Adhesins, Regulation, and Host Response. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the Natural environment to infectious diseases. Nat. Rev. Microbiol 2004, 2, 95. [Google Scholar] [CrossRef]
- Kumar, A.; Alam, A.; Rani, M.; Ehtesham, N.Z.; Hasnain, S.E. Biofilms: Survival and defense strategy for pathogens. Int. J. Med. Microbiol. 2017, 307, 481–489. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Ferreira Tde, O.; Koto, R.Y.; Leite, G.F.; Klautau, G.B.; Nigro, S.; Silva, C.B.; Souza, A.P.; Mimica, M.J.; Cesar, R.G.; Salles, M.J. Microbial investigation of biofilms recovered from endotracheal tubes using sonication in intensive care unit pediatric patients. Braz. J. Infect. Dis. Off. Publ. Braz. Soc. Infect. Dis. 2016, 20, 468–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardes, J.M.; Waters, C.; Motlagh, H.; Wilson, A. The Prevalence of Oral Flora in the Biofilm Microbiota of the Endotracheal Tube. Am. Surg. 2016, 82, 403–406. [Google Scholar] [CrossRef]
- Li, H.; Song, C.; Liu, D.; Ai, Q.; Yu, J. Molecular analysis of biofilms on the surface of neonatal endotracheal tubes based on 16S rRNA PCR-DGGE and species-specific PCR. Int. J. Clin. Exp. Med. 2015, 8, 11075–11084. [Google Scholar] [PubMed]
- Vandecandelaere, I.; Matthijs, N.; Van Nieuwerburgh, F.; Deforce, D.; Vosters, P.; De Bus, L.; Nelis, H.J.; Depuydt, P.; Coenye, T. Assessment of microbial diversity in biofilms recovered from endotracheal tubes using culture dependent and independent approaches. PLoS ONE 2012, 7, e38401. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Lau, E.; Watson, H.; Schmier, J.K.; Parvizi, J. Economic burden of periprosthetic joint infection in the United States. J. Arthroplast. 2012, 27, 61–65.e1. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, M.G.; Meaike, J.D.; Izaddoost, S.A. Orthopedic Prosthetic Infections: Diagnosis and Orthopedic Salvage. Semin. Plast. Surg. 2016, 30, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Arciola, C.R.; Campoccia, D.; Ehrlich, G.D.; Montanaro, L. Biofilm-based implant infections in orthopaedics. Adv. Exp. Med. Biol. 2015, 830, 29–46. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants—A review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef]
- Gristina, A.G.; Naylor, P.T.; Myrvik, Q. The Race for the Surface: Microbes, Tissue Cells, and Biomaterials. In Molecular Mechanisms of Microbial Adhesion; Springer: New York, NY, USA, 1989; pp. 177–211. [Google Scholar]
- Perez-Tanoira, R.; Han, X.; Soininen, A.; Aarnisalo, A.A.; Tiainen, V.M.; Eklund, K.K.; Esteban, J.; Kinnari, T.J. Competitive colonization of prosthetic surfaces by Staphylococcus aureus and human cells. J. Biomed. Mater. Res. Part A 2017, 105, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Stones, D.H.; Krachler, A.M. Against the tide: The role of bacterial adhesion in host colonization. Biochem. Soc. Trans. 2016, 44, 1571–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaskiewicz, M.; Janczura, A.; Nowicka, J.; Kamysz, W. Methods Used for the Eradication of Staphylococcal Biofilms. Antibiotics 2019, 8, 174. [Google Scholar] [CrossRef] [Green Version]
- Reigada, I.; Perez-Tanoira, R.; Patel, J.Z.; Savijoki, K.; Yli-Kauhaluoma, J.; Kinnari, T.J.; Fallarero, A. Strategies to Prevent Biofilm Infections on Biomaterials: Effect of Novel Naturally-Derived Biofilm Inhibitors on a Competitive Colonization Model of Titanium by Staphylococcus aureus and SaOS-2 Cells. Microorganisms 2020, 8, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manner, S.; Vahermo, M.; Skogman, M.E.; Krogerus, S.; Vuorela, P.M.; Yli-Kauhaluoma, J.; Fallarero, A.; Moreira, V.M. New derivatives of dehydroabietic acid target planktonic and biofilm bacteria in Staphylococcus aureus and effectively disrupt bacterial membrane integrity. Eur. J. Med. Chem. 2015, 102, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Manner, S.; Skogman, M.; Goeres, D.; Vuorela, P.; Fallarero, A. Systematic Exploration of Natural and Synthetic Flavonoids for the Inhibition of Staphylococcus aureus Biofilms. Int. J. Mol. Sci. 2013, 14, 19434–19451. [Google Scholar] [CrossRef] [Green Version]
- Jhunjhunwala, S. Neutrophils at the Biological–Material Interface. ACS Biomater. Sci. Eng. 2018, 4, 1128–1136. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Kolodkin-Gal, I.; Foulston, L.; Kolter, R.; Aizenberg, J.; Losick, R. Inhibitory effects of D-amino acids on Staphylococcus aureus biofilm development. J. Bacteriol. 2011, 193, 5616–5622. [Google Scholar] [CrossRef] [Green Version]
- Esteban, J.; Gomez-Barrena, E.; Cordero, J.; Martin-de-Hijas, N.Z.; Kinnari, T.J.; Fernandez-Roblas, R. Evaluation of quantitative analysis of cultures from sonicated retrieved orthopedic implants in diagnosis of orthopedic infection. J. Clin. Microbiol. 2008, 46, 488–492. [Google Scholar] [CrossRef] [Green Version]
- Collins, S.J.; Ruscetti, F.W.; Gallagher, R.E.; Gallo, R.C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc. Natl. Acad. Sci. USA 1978, 75, 2458–2462. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Porter, A.R.; Kennedy, A.D.; Kobayashi, S.D.; DeLeo, F.R. Phagocytosis and killing of Staphylococcus aureus by human neutrophils. J. Innate Immun. 2014, 6, 639–649. [Google Scholar] [CrossRef]
- van Kessel, K.P.M.; Bestebroer, J.; van Strijp, J.A.G. Neutrophil-Mediated Phagocytosis of Staphylococcus aureus. Front. Immunol. 2014, 5, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiltunen, A.K.; Savijoki, K.; Nyman, T.A.; Miettinen, I.; Ihalainen, P.; Peltonen, J.; Fallarero, A. Structural and Functional Dynamics of Staphylococcus aureus Biofilms and Biofilm Matrix Proteins on Different Clinical Materials. Microorganisms 2019, 7, 584. [Google Scholar] [CrossRef] [Green Version]
- Sunzel, B.; Söderberg, T.A.; Reuterving, C.O.; Hallmans, G.; Holm, S.E.; Hänström, L. Neutralizing effect of zinc oxide on dehydroabietic acid-induced toxicity on human polymorphonuclear leukocytes. Biol. Trace Elem. 1991, 30, 257–266. [Google Scholar] [CrossRef]
- Czerwińska, M.E.; Dudek, M.K.; Pawłowska, K.A.; Pruś, A.; Ziaja, M.; Granica, S. The influence of procyanidins isolated from small-leaved lime flowers (Tilia cordata Mill.) on human neutrophils. Fitoterapia 2018, 127, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, K.A.; Hałasa, R.; Dudek, M.K.; Majdan, M.; Jankowska, K.; Granica, S. Antibacterial and anti-inflammatory activity of bistort (Bistorta officinalis) aqueous extract and its major components. Justification of the usage of the medicinal plant material as a traditional topical agent. J. Ethnopharmacol. 2020, 260, 113077. [Google Scholar] [CrossRef]
- Manda-Handzlik, A.; Bystrzycka, W.; Wachowska, M.; Sieczkowska, S.; Stelmaszczyk-Emmel, A.; Demkow, U.; Ciepiela, O. The influence of agents differentiating HL-60 cells toward granulocyte-like cells on their ability to release neutrophil extracellular traps. Immunol. Cell Biol. 2018, 96, 413–425. [Google Scholar] [CrossRef]
- Yaseen, R.; Blodkamp, S.; Luthje, P.; Reuner, F.; Vollger, L.; Naim, H.Y.; von Kockritz-Blickwede, M. Antimicrobial activity of HL-60 cells compared to primary blood-derived neutrophils against Staphylococcus aureus. J. Negat. Results Biomed. 2017, 16, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croes, M.; Bakhshandeh, S.; van Hengel, I.A.J.; Lietaert, K.; van Kessel, K.P.M.; Pouran, B.; van der Wal, B.C.H.; Vogely, H.C.; Van Hecke, W.; Fluit, A.C.; et al. Antibacterial and immunogenic behavior of silver coatings on additively manufactured porous titanium. Acta Biomater. 2018, 81, 315–327. [Google Scholar] [CrossRef]
- Seitz, A.P.; Schumacher, F.; Baker, J.; Soddemann, M.; Wilker, B.; Caldwell, C.C.; Gobble, R.M.; Kamler, M.; Becker, K.A.; Beck, S.; et al. Sphingosine-coating of plastic surfaces prevents ventilator-associated pneumonia. J. Mol. Med. 2019, 97, 1195–1211. [Google Scholar] [CrossRef] [Green Version]
- Lyczak, J.B.; Cannon, C.L.; Pier, G.B. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 2002, 15, 194–222. [Google Scholar] [CrossRef] [Green Version]
- Alves, P.M.; Al-Badi, E.; Withycombe, C.; Jones, P.M.; Purdy, K.J.; Maddocks, S.E. Interaction between Staphylococcus aureus and Pseudomonas aeruginosa is beneficial for colonisation and pathogenicity in a mixed biofilm. Pathog. Dis. 2018, 76. [Google Scholar] [CrossRef]
- Beaudoin, T.; Yau, Y.C.W.; Stapleton, P.J.; Gong, Y.; Wang, P.W.; Guttman, D.S.; Waters, V. Staphylococcus aureus interaction with Pseudomonas aeruginosa biofilm enhances tobramycin resistance. NPJ Biofilms Microbiomes 2017, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chu, L.; Yang, S.; Zhang, H.; Qin, L.; Guillaume, O.; Eglin, D.; Richards, R.G.; Tang, T. Dual-functional 3D-printed composite scaffold for inhibiting bacterial infection and promoting bone regeneration in infected bone defect models. Acta Biomater. 2018, 79, 265–275. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reigada, I.; Guarch-Pérez, C.; Patel, J.Z.; Riool, M.; Savijoki, K.; Yli-Kauhaluoma, J.; Zaat, S.A.J.; Fallarero, A. Combined Effect of Naturally-Derived Biofilm Inhibitors and Differentiated HL-60 Cells in the Prevention of Staphylococcus aureus Biofilm Formation. Microorganisms 2020, 8, 1757. https://doi.org/10.3390/microorganisms8111757
Reigada I, Guarch-Pérez C, Patel JZ, Riool M, Savijoki K, Yli-Kauhaluoma J, Zaat SAJ, Fallarero A. Combined Effect of Naturally-Derived Biofilm Inhibitors and Differentiated HL-60 Cells in the Prevention of Staphylococcus aureus Biofilm Formation. Microorganisms. 2020; 8(11):1757. https://doi.org/10.3390/microorganisms8111757
Chicago/Turabian StyleReigada, Inés, Clara Guarch-Pérez, Jayendra Z. Patel, Martijn Riool, Kirsi Savijoki, Jari Yli-Kauhaluoma, Sebastian A. J. Zaat, and Adyary Fallarero. 2020. "Combined Effect of Naturally-Derived Biofilm Inhibitors and Differentiated HL-60 Cells in the Prevention of Staphylococcus aureus Biofilm Formation" Microorganisms 8, no. 11: 1757. https://doi.org/10.3390/microorganisms8111757
APA StyleReigada, I., Guarch-Pérez, C., Patel, J. Z., Riool, M., Savijoki, K., Yli-Kauhaluoma, J., Zaat, S. A. J., & Fallarero, A. (2020). Combined Effect of Naturally-Derived Biofilm Inhibitors and Differentiated HL-60 Cells in the Prevention of Staphylococcus aureus Biofilm Formation. Microorganisms, 8(11), 1757. https://doi.org/10.3390/microorganisms8111757