Serodiagnosis of Visceral Leishmaniasis in Northeastern Italy: Evaluation of Seven Serological Tests
Abstract
:1. Introduction
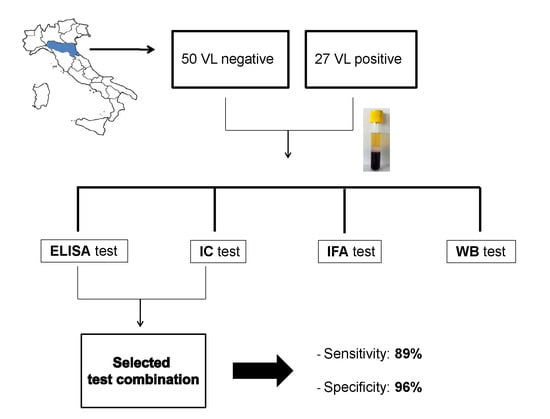
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Control of the Leishmaniases: Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22–26 March 2010. 2010. Available online: https://apps.who.int/iris/bitstream/handle/10665/44412/WHO_TRS_949_eng.pdf;jsessionid=C8F6BF44C88D370A5B47380A742D17E9?sequence=1 (accessed on 24 October 2020).
- Gradoni, R.L.; Mokni, M. Manual on Case Management and Surveillance of the Leishmaniases in the WHO European Region. 2017. Available online: https://www.euro.who.int/__data/assets/pdf_file/0006/341970/MANUAL-ON-CASE-MANAGEMENT_FINAL_with-cover-and-ISBN.pdf (accessed on 24 October 2020).
- Horrillo, L.; Castro, A.; Matía, B.; Molina, L.; García-Martínez, J.; Jaqueti, J.; García-Arata, I.; Carrillo, E.; Moreno, J.; Ruiz-Giardin, J.M.; et al. Clinical aspects of visceral leishmaniasis caused by L. infantum in adults. Ten years of experience of the largest outbreak in Europe: What have we learned? Parasit Vectors 2019, 12, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varani, S.; Cagarelli, R.; Melchionda, F.; Attard, L.; Salvadori, C.; Finarelli, A.C.; Gentilomi, G.A.; Tigani, R.; Rangoni, R.; Todeschini, R.; et al. Ongoing outbreak of visceral leishmaniasis in Bologna Province, Italy, November 2012 to May 2013. Eurosurveillance 2013, 18, 20530. [Google Scholar] [CrossRef] [Green Version]
- Franceschini, E.; Puzzolante, C.; Menozzi, M.; Rossi, L.; Bedini, A.; Orlando, G.; Gennari, W.; Meacci, M.; Rugna, G.; Carra, E.; et al. Clinical and Microbiological Characteristics of Visceral Leishmaniasis Outbreak in a Northern Italian Nonendemic Area: A Retrospective Observational Study. Biomed. Res. Int. 2016, 2016, 6481028. [Google Scholar] [CrossRef] [PubMed]
- Babuadze, G.; Alvar, J.; Argaw, D.; de Koning, H.P.; Iosava, M.; Kekelidze, M.; Tsertsvadze, N.; Tsereteli, D.; Chakhunashvili, G.; Mamatsashvili, T.; et al. Epidemiology of visceral leishmaniasis in Georgia. PLoS Negl. Trop. Dis. 2014, 8, e2725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aronson, N.; Herwaldt, B.L.; Libman, M.; Pearson, R.; Lopez-Velez, R.; Weina, P.; Carvalho, E.M.; Ephros, M.; Jeronimo, S.; Magill, A. Diagnosis and Treatment of Leishmaniasis: Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin. Infect. Dis. 2016, 63, e202–e264. [Google Scholar] [CrossRef] [PubMed]
- Van Griensven, J.; Diro, E. Visceral Leishmaniasis: Recent Advances in Diagnostics and Treatment Regimens. Infect. Dis. Clin. N. Am. 2019, 33, 79–99. [Google Scholar] [CrossRef]
- Elmahallawy, E.K.; Sampedro Martinez, A.; Rodriguez-Granger, J.; Hoyos-Mallecot, Y.; Agil, A.; Navarro Mari, J.M.; Gutierrez Fernandez, J. Diagnosis of leishmaniasis. J. Infect. Dev. Ctries 2014, 8, 961–972. [Google Scholar] [CrossRef]
- Chappuis, F.; Sundar, S.; Hailu, A.; Ghalib, H.; Rijal, S.; Peeling, R.W.; Alvar, J.; Boelaert, M. Visceral leishmaniasis: What are the needs for diagnosis, treatment and control? Nat. Rev. Microbiol. 2007, 5, 873–882. [Google Scholar] [CrossRef]
- Boelaert, M.; Verdonck, K.; Menten, J.; Sunyoto, T.; van Griensven, J.; Chappuis, F.; Rijal, S. Rapid tests for the diagnosis of visceral leishmaniasis in patients with suspected disease. Cochrane Database Syst. Rev. 2014, 6, CD009135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varani, S.; Ortalli, M.; Attard, L.; Vanino, E.; Gaibani, P.; Vocale, C.; Rossini, G.; Cagarelli, R.; Pierro, A.; Billi, P.; et al. Serological and molecular tools to diagnose visceral leishmaniasis: 2-years’ experience of a single center in Northern Italy. PLoS ONE 2017, 12, e0183699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mary, C.; Lamouroux, D.; Dunan, S.; Quilici, M. Western blot analysis of antibodies to Leishmania infantum antigens: Potential of the 14-kD and 16-kD antigens for diagnosis and epidemiologic purposes. Am. J. Trop. Med. Hyg. 1992, 47, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Maia, Z.; Lírio, M.; Mistro, S.; Mendes, C.M.; Mehta, S.R.; Badaro, R. Comparative study of rK39 Leishmania antigen for serodiagnosis of visceral leishmaniasis: Systematic review with meta-analysis. PLoS Negl. Trop. Dis. 2012, 6, e1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bangert, M.; Flores-Chávez, M.D.; Llanes-Acevedo, I.P.; Arcones, C.; Chicharro, C.; García, E.; Ortega, S.; Nieto, J.; Cruz, I. Validation of rK39 immunochromatographic test and direct agglutination test for the diagnosis of Mediterranean visceral leishmaniasis in Spain. PLoS Negl. Trop. Dis. 2018, 12, e0006277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lévêque, M.F.; Battery, E.; Delaunay, P.; Lmimouni, B.E.; Aoun, K.; L’Ollivier, C.; Bastien, P.; Mary, C.; Pomares, C.; Fillaux, J.; et al. Evaluation of six commercial kits for the serological diagnosis of Mediterranean visceral leishmaniasis. PLoS Negl. Trop. Dis. 2020, 14, e0008139. [Google Scholar] [CrossRef] [PubMed]
- Bezuneh, A.; Mukhtar, M.; Abdoun, A.; Teferi, T.; Takele, Y.; Diro, E.; Jemaneh, A.; Shiferaw, W.; Wondimu, H.; Bhatia, A.; et al. Comparison of point-of-care tests for the rapid diagnosis of visceral leishmaniasis in East African patients. Am. J. Trop. Med. Hyg. 2014, 91, 1109–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abass, E.; Kang, C.; Martinkovic, F.; Semião-Santos, S.J.; Sundar, S.; Walden, P.; Piarroux, R.; El Harith, A.; Lohoff, M.; Steinhoff, U. Heterogeneity of Leishmania donovani Parasites Complicates Diagnosis of Visceral Leishmaniasis: Comparison of Different Serological Tests in Three Endemic Regions. PLoS ONE 2015, 10, e0116408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO/TDR. Visceral Leishmaniasis Rapid Diagnostic Test Performance. 2011. Available online: https://www.who.int/tdr/publications/documents/vl-rdt-evaluation.pdf (accessed on 19 November 2020).
- Akuffo, H.; Costa, C.; van Griensven, J.; Burza, S.; Moreno, J.; Herrero, M. New insights into leishmaniasis in the immunosuppressed. PLoS Negl. Trop. Dis. 2018, 12, e0006375. [Google Scholar] [CrossRef] [PubMed]
- Biglino, A.; Bolla, C.; Concialdi, E.; Trisciuoglio, A.; Romano, A.; Ferroglio, E. Asymptomatic Leishmania infantum infection in an area of northwestern Italy (Piedmont region) where such infections are traditionally nonendemic. J. Clin. Microbiol. 2010, 48, 131–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gramiccia, M.; Scalone, A.; Di Muccio, T.; Orsini, S.; Fiorentino, E.; Gradoni, L. The burden of visceral leishmaniasis in Italy from 1982 to 2012: A retrospective analysis of the multi-annual epidemic that occurred from 1989 to 2009. Eurosurveillance 2013, 18, 20535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| TEST | Sensitivity % | Specificity % | PPV | NPV | Accuracy % |
|---|---|---|---|---|---|
| Apacor ICT | 70 (50–85) | 96 (85–99) | 90 (68–98) | 86 (73–93) | 87 |
| CTK Biotech ICT | 63 (42–80) | 98 (88–100) | 94 (71–100) | 83 (70–91) | 86 |
| Cypress ICT | 52 (32–71) | 100 (91–100) | 100 (73–100) | 79 (67–88) | 83 |
| Vircell ELISA | 74 (53–88) | 98 (88–100) | 95 (74–100) | 88 (75–94) | 90 |
| NovaTec ELISA | 63 (42–80) | 100 (91–100) | 100 (77–100) | 83 (71–91) | 87 |
| IFAT | 96 (80–100) | 100 (91–100) | 100 (84–100) | 98 (88–100) | 99 |
| WB | 96 (80–100) | 88 (75–95) | 81 (63–92) | 98 (87–100) | 91 |
| Test Combination | Sensitivity % | Specificity % |
|---|---|---|
| ELISA Vircell + Apacor ICT | 89 (70–97) | 94 (82–98) |
| ELISA Vircell + Cypress ICT | 82 (61–93) | 98 (88–100) |
| ELISA Vircell + CTK Biotech ICT | 89 (70–97) | 96 (85–99) |
| NovaTec ELISA + Apacor ICT | 85 (65–95) | 96 (85–99) |
| NovaTec ELISA+ Cypress ICT | 82 (61–93) | 100 (91–100) |
| NovaTec ELISA+ CTK Biotech ICT | 82 (61–93) | 98 (88–100) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortalli, M.; Lorrai, D.; Gaibani, P.; Rossini, G.; Vocale, C.; Re, M.C.; Varani, S. Serodiagnosis of Visceral Leishmaniasis in Northeastern Italy: Evaluation of Seven Serological Tests. Microorganisms 2020, 8, 1847. https://doi.org/10.3390/microorganisms8121847
Ortalli M, Lorrai D, Gaibani P, Rossini G, Vocale C, Re MC, Varani S. Serodiagnosis of Visceral Leishmaniasis in Northeastern Italy: Evaluation of Seven Serological Tests. Microorganisms. 2020; 8(12):1847. https://doi.org/10.3390/microorganisms8121847
Chicago/Turabian StyleOrtalli, Margherita, Daniele Lorrai, Paolo Gaibani, Giada Rossini, Caterina Vocale, Maria Carla Re, and Stefania Varani. 2020. "Serodiagnosis of Visceral Leishmaniasis in Northeastern Italy: Evaluation of Seven Serological Tests" Microorganisms 8, no. 12: 1847. https://doi.org/10.3390/microorganisms8121847
APA StyleOrtalli, M., Lorrai, D., Gaibani, P., Rossini, G., Vocale, C., Re, M. C., & Varani, S. (2020). Serodiagnosis of Visceral Leishmaniasis in Northeastern Italy: Evaluation of Seven Serological Tests. Microorganisms, 8(12), 1847. https://doi.org/10.3390/microorganisms8121847