Do Foliar Endophytes Matter in Litter Decomposition?
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Endophytes and Litter Chemistry
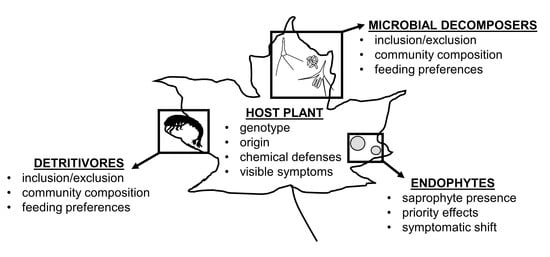
4.2. Endophytes as Decomposers (Interactions with Detritivores and Microbial Decomposers)
4.3. Endophytes in Litter Decomposer Assemblages
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Boyero, L.; Pearson, R.G.; Gessner, M.O.; Barmuta, L.A.; Ferreira, V.; Graça, M.A.S.; Dudgeon, D.; Boulton, A.J.; Callisto, M.; Chauvet, E.; et al. A global experiment suggests climate warming will not accelerate litter decomposition in streams but might reduce carbon sequestration. Ecol. Lett. 2011, 14, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Gosz, J.R.; Likens, G.E.; Bormann, F.H. Nutrient release from decomposing leaf and branch litter in the hubbard brook forest, new hampshire. Ecol. Monogr. 1973, 43, 173. [Google Scholar] [CrossRef]
- Medeiros, A.O.; Pascoal, C.; Graça, M.A.S. Diversity and activity of aquatic fungi under low oxygen conditions. Freshw. Biol. 2009, 54, 142–149. [Google Scholar] [CrossRef] [Green Version]
- LeRoy, C.J.; Wymore, A.S.; Davis, R.; Marks, J.C. Indirect influences of a major drought on leaf litter quality and decomposition in a southwestern stream. Fundam. Appl. Limnol. Arch. Für Hydrobiol. 2014, 184, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ballhorn, D.J.; Elias, J.D.; Balkan, M.A.; Fordyce, R.F.; Kennedy, P.G. Colonization by nitrogen-fixing Frankia bacteria causes short-term increases in herbivore susceptibility in red alder (Alnus rubra) seedlings. Oecologia 2017, 184, 497–506. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Kay, J.; Kautz, S. Quantitative effects of leaf area removal on indirect defense of lima bean (Phaseolus lunatus) in nature. J. Chem. Ecol. 2014, 40, 294–296. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Kautz, S.; Schädler, M. Induced plant defense via volatile production is dependent on rhizobial symbiosis. Oecologia 2013, 172, 833–846. [Google Scholar] [CrossRef]
- Pańka, D.; Piesik, D.; Jeske, M.; Baturo-Cieśniewska, A. Production of phenolics and the emission of volatile organic compounds by perennial ryegrass (Lolium perenne L.)/Neotyphodium lolii association as a response to infection by Fusarium poae. J. Plant Physiol. 2013, 170, 1010–1019. [Google Scholar] [CrossRef]
- Chomel, M.; Guittonny-Larchevêque, M.; Fernandez, C.; Gallet, C.; DesRochers, A.; Paré, D.; Jackson, B.G.; Baldy, V. Plant secondary metabolites: A key driver of litter decomposition and soil nutrient cycling. J. Ecol. 2016, 104, 1527–1541. [Google Scholar] [CrossRef]
- Stone, J.K.; Polishook, J.O.N.D.; White, J.F. Endophytic fungi. In Measuring and Monitoring Biodiversity of Fungi; Mueller, G., Bills, G., Foster, M., Eds.; Elsevier Academic Press: Boston, MA, USA, 2004; pp. 241–270. ISBN 978-0-12-509551-8. [Google Scholar]
- Rodriguez, R.J.; White, J.F.; Arnold, A.E.; Redman, R.S. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef]
- Bacon, C.W. Procedure for isolating the endophyte from tall fescue and screening isolates for ergot alkaloids. Appl. Environ. Microbiol. 1988, 54, 2615–2618. [Google Scholar] [CrossRef] [Green Version]
- Rudgers, J.A.; Holah, J.; Orr, S.P.; Clay, K.; Orr, P. Forest succession suppressed by an introduced plant-fungal symbiosis. Ecol. Soc. Am. 2007, 88, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Rudgers, J.A.; Clay, K. Endophyte symbiosis with tall fescue: How strong are the impacts on communities and ecosystems? Fungal Biol. Rev. 2007, 21, 107–124. [Google Scholar] [CrossRef]
- Lemons, A.; Clay, K.; Rudgers, J.A. Connecting plant-microbial interactions above and belowground: A fungal endophyte affects decomposition. Oecologia 2005, 145, 595–604. [Google Scholar] [CrossRef]
- Siegrist, J.A.; McCulley, R.L.; Bush, L.P.; Phillips, T.D. Alkaloids may not be responsible for endophyte-associated reductions in tall fescue decomposition rates. Funct. Ecol. 2010, 24, 460–468. [Google Scholar] [CrossRef]
- Younginger, B.S.; Ballhorn, D.J. Fungal endophyte communities in the temperate fern Polystichum munitum show early colonization and extensive temporal turnover. Am. J. Bot. 2017, 104, 1188–1194. [Google Scholar] [CrossRef] [Green Version]
- Osono, T. Role of phyllosphere fungi of forest trees in the development of decomposer fungal communities and decomposition processes of leaf litter. Can. J. Microbiol. 2006, 52, 701–716. [Google Scholar] [CrossRef]
- Anderson, J.M.; Hetherington, S.L. Temperature, nitrogen availability and mixture effects on the decomposition of heather [Calluna vulgaris (L.) Hull] and bracken [Pteridium aquilinum (L.) Kuhn] litters. Funct. Ecol. 1999, 13, 116–124. [Google Scholar] [CrossRef]
- Friesen, M.L.; Porter, S.S.; Stark, S.C.; von Wettberg, E.J.; Sachs, J.L.; Martinez-Romero, E. Microbially Mediated Plant Functional Traits. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 23–46. [Google Scholar] [CrossRef] [Green Version]
- Wurst, S.; Ohgushi, T. Do plant- and soil-mediated legacy effects impact future biotic interactions? Funct. Ecol. 2015, 29, 1373–1382. [Google Scholar] [CrossRef]
- Buyer, J.S.; Zuberer, D.A.; Nichols, K.A.; Franzluebbers, A.J. Soil microbial community function, structure, and glomalin in response to tall fescue endophyte infection. Plant Soil 2011, 339, 401–412. [Google Scholar] [CrossRef]
- Guo, J.; McCulley, R.L.; Phillips, T.D.; McNear, D.H. Fungal endophyte and tall fescue cultivar interact to differentially affect bulk and rhizosphere soil processes governing C and N cycling. Soil Biol. Biochem. 2016, 101, 165–174. [Google Scholar] [CrossRef]
- Iqbal, J.; Siegrist, J.A.; Nelson, J.A.; McCulley, R.L. Fungal endophyte infection increases carbon sequestration potential of southeastern USA tall fescue stands. Soil Biol. Biochem. 2012, 44, 81–92. [Google Scholar] [CrossRef]
- Hosseini, F.; Mosaddeghi, M.R.; Hajabbasi, M.A.; Mamedov, A.I. Effects of endophyte-infected and non-infected tall fescue residues on aggregate stability in four texturally different soils. Geoderma 2017, 285, 195–205. [Google Scholar] [CrossRef]
- Osono, T.; Takeda, H. Decomposing ability of interior and surface fungal colonizers of beech leaves with reference to lignin decomposition. Eur. J. Soil Biol. 1999, 35, 51–56. [Google Scholar] [CrossRef]
- Osono, T. Phyllosphere fungi on leaf litter of Fagus crenata: Occurrence, colonization, and succession. Can. J. Bot. 2002, 80, 460–469. [Google Scholar] [CrossRef]
- Wolfe, E.R.; Younginger, B.S.; LeRoy, C.J. Fungal endophyte-infected leaf litter alters in-stream microbial communities and negatively influences aquatic fungal sporulation. Oikos 2019, 128, 405–415. [Google Scholar] [CrossRef]
- LeRoy, C.J.; Fischer, D.G.; Halstead, K.; Pryor, M.; Bailey, J.K.; Schweitzer, J.A. A fungal endophyte slows litter decomposition in streams. Freshw. Biol. 2011, 56, 1426–1433. [Google Scholar] [CrossRef]
- Guerreiro, M.A.; Brachmann, A.; Dominik, B.; Peršoh, D. Transient leaf endophytes are the most active fungi in 1-year-old beech leaf litter. Fungal Divers. 2018, 89, 237–251. [Google Scholar] [CrossRef] [Green Version]
- Stone, J.K.; Sherwood, M.A.; Carroll, G.C. Canopy microfungi: Function and diversity. Northwest Sci. 1996, 70, 37–45. [Google Scholar]
- Osono, T. Endophytic and epiphytic phyllosphere fungi of Camellia japonica: Seasonal and leaf age-dependent variations. Mycologia 2008, 100, 387–391. [Google Scholar] [CrossRef]
- Osono, T.; Hirose, D. Effects of prior decomposition of Camellia japonica leaf litter by an endophytic fungus on the subsequent decomposition by fungal colonizers. Mycoscience 2009, 50, 52–55. [Google Scholar] [CrossRef]
- Osono, T.; Tateno, O.; Masuya, H. Diversity and ubiquity of xylariaceous endophytes in live and dead leaves of temperate forest trees. Mycoscience 2013, 54, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Tateno, O.; Hirose, D.; Osono, T.; Takeda, H. Beech cupules share endophytic fungi with leaves and twigs. Mycoscience 2015, 56, 252–256. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Lavina, C.X.; Bettucci, L.; Tiscornia, S. Fungal communities associated with Eugenia uruguayensis (Myrtaceae) leaf litter in early stages of decomposition in uruguay. Sydowia 2016, 68, 139–150. [Google Scholar]
- Szink, I.; Davis, E.L.; Ricks, K.D.; Koide, R.T. New evidence for broad trophic status of leaf endophytic fungi of Quercus gambelii. Fungal Ecol. 2016, 22, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Grimmett, I.J.; Smith, K.A.; Bärlocher, F. Tar-spot infection delays fungal colonization and decomposition of maple leaves. Freshw. Sci. 2012, 31, 1088–1095. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, E.R.; Kautz, S.; Singleton, S.L.; Ballhorn, D.J. Differences in foliar endophyte communities of red alder (Alnus rubra) exposed to varying air pollutant levels. Botany 2018, 96, 825–835. [Google Scholar] [CrossRef]
- Paulus, B.C.; Kanowski, J.; Gadek, P.A.; Hyde, K.D. Diversity and distribution of saprobic microfungi in leaf litter of an Australian tropical rainforest. Mycol. Res. 2006, 110, 1441–1454. [Google Scholar] [CrossRef]
- Polishook, J.D.; Bills, G.F.; Lodge, D.J. Microfungi from decaying leaves of two rain forest trees in Puerto Rico. J. Ind. Microbiol. Biotechnol. 1996, 17, 284–294. [Google Scholar] [CrossRef]
- Bills, G.F.; Polishook, J.D. Abundance and diversity of microfungi in leaf litter of a lowland rain forest in costa rica. Mycologia 1994, 86, 187–198. [Google Scholar] [CrossRef]
- Promputtha, I.; Hyde, K.D.; McKenzie, E.H.C.; Peberdy, J.F.; Lumyong, S. Can leaf degrading enzymes provide evidence that endophytic fungi becoming saprobes? Fungal Divers. 2010, 41, 89–99. [Google Scholar] [CrossRef]
- Hirose, D.; Matsuoka, S.; Osono, T. Assessment of the fungal diversity and succession of ligninolytic endophytes in Camellia japonica leaves using clone library analysis. Mycologia 2013, 105, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Prakash, C.P.; Thirumalai, E.; Govinda Rajulu, M.B.; Thirunavukkarasu, N.; Suryanarayanan, T.S. Ecology and diversity of leaf litter fungi during early-stage decomposition in a seasonally dry tropical forest. Fungal Ecol. 2015, 17, 103–113. [Google Scholar] [CrossRef]
- Murugaiyan, K. Marine fungal diversity and bioprospecting. In Springer Handbook of Marine Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 13–25. ISBN 9783642539718. [Google Scholar]
- Mustonen, K.-R.; Mykrä, H.; Louhi, P.; Markkola, A.; Tolkkinen, M.; Huusko, A.; Alioravainen, N.; Lehtinen, S.; Muotka, T. Sediments and flow have mainly independent effects on multitrophic stream communities and ecosystem functions. Ecol. Appl. 2016, 26, 2116–2129. [Google Scholar] [CrossRef]
- Hagiwara, Y.; Matsuoka, S.; Hobara, S.; Mori, A.S.; Hirose, D.; Osono, T. Bleaching of leaf litter and associated microfungi in subboreal and subalpine forests. Can. J. Microbiol. 2015, 61, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Korkama-Rajala, T.; Müller, M.M.; Pennanen, T. Decomposition and Fungi of Needle Litter from Slow- and Fast-growing Norway Spruce (Picea abies) Clones. Microb. Ecol. 2008, 56, 76–89. [Google Scholar] [CrossRef]
- Przybył, K.; Karolewski, P.; Oleksyn, J.; Łabȩdzki, A.; Reich, P.B. Fungal diversity of Norway spruce litter: Effects of site conditions and premature leaf fall caused by bark beetle outbreak. Microb. Ecol. 2008, 56, 332–340. [Google Scholar] [CrossRef]
- Žifčáková, L.; Dobiášová, P.; Kolářová, Z.; Koukol, O. Enzyme activities of fungi associated with Picea abies needles. Fungal Ecol. 2011, 4, 427–436. [Google Scholar] [CrossRef]
- Unterseher, M.; Peršoh, D.; Schnittler, M. Leaf-inhabiting endophytic fungi of european beech (Fagus sylvatica L.) co-occur in leaf litter but are rare on decaying wood of the same host. Fungal Divers. 2013, 60, 43–54. [Google Scholar] [CrossRef]
- Voříšková, J.; Baldrian, P. Fungal community on decomposing leaf litter undergoes rapid successional changes. ISME J. 2013, 7, 477–486. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Han, G.; Lin, Y.; Tian, X.; Xiang, C.; Tian, Q.; Wang, F.; He, Z. Diversity and decomposition potential of endophytes in leaves of a Cinnamomum camphora plantation in China. Ecol. Res. 2012, 27, 273–284. [Google Scholar] [CrossRef]
- Mayer, P.M.; Tunnell, S.J.; Engle, D.M.; Jorgensen, E.E.; Nunn, P. Invasive grass alters litter decomposition by influencing macrodetritivores. Ecosystems 2005, 8, 200–209. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.-W.; Li, L.; Dai, C.-C. The potential application of the endophyte Phomopsis liquidambari to the ecological remediation of long-term cropping soil. Appl. Soil Ecol. 2013, 67, 20–26. [Google Scholar] [CrossRef]
- Lin, Y.; He, X.; Ma, T.; Han, G.; Xiang, C. Priority colonization of Cinnamomum camphora litter by endophytes affects decomposition rate, fungal community and microbial activities under field conditions. Pedobiologia 2015, 58, 177–185. [Google Scholar] [CrossRef]
- Gundel, P.E.; Helander, M.; Garibaldi, L.A.; Vázquez-de-Aldana, B.R.; Zabalgogeazcoa, I.; Saikkonen, K. Direct and indirect effects of the fungal endophyte Epichloë uncinatum on litter decomposition of the host grass, Schedonorus pratensis. Plant Ecol. 2017, 218, 1107–1115. [Google Scholar] [CrossRef]
- Gundel, P.E.; Helander, M.; Garibaldi, L.A.; Vázquez-de-Aldana, B.R.; Zabalgogeazcoa, I.; Saikkonen, K. Role of foliar fungal endophytes in litter decomposition among species and population origins. Fungal Ecol. 2016, 21, 50–56. [Google Scholar] [CrossRef]
- Sun, K.; Cao, W.; Hu, L.Y.; Fu, W.Q.; Gong, J.H.; Kang, N.; Dai, C.C. Symbiotic fungal endophyte Phomopsis liquidambari-rice system promotes nitrogen transformation by influencing below-ground straw decomposition in paddy soil. J. Appl. Microbiol. 2019, 126, 191–203. [Google Scholar] [CrossRef]
- Bell-Dereske, L.; Gao, X.; Masiello, C.A.; Sinsabaugh, R.L.; Emery, S.M.; Rudgers, J.A. Plant–fungal symbiosis affects litter decomposition during primary succession. Oikos 2017, 126, 801–811. [Google Scholar] [CrossRef]
- Omacini, M.; Chaneton, J.E.; Ghersa, C.M.; Otero, P. Do foliar endophytes affect grass litter decomposition? A microcosm approach using Lolium multiflorum. Oikos 2004, 104, 581–590. [Google Scholar] [CrossRef]
- Omacini, M.; Semmartin, M.; Pérez, L.I.; Gundel, P.E. Grass–endophyte symbiosis: A neglected aboveground interaction with multiple belowground consequences. Appl. Soil Ecol. 2012, 61, 273–279. [Google Scholar] [CrossRef]
- Mikola, J.; Helander, M.; Saikkonen, K. No effects of Epichloë endophyte infection on nitrogen cycling in meadow fescue (Schedonorus pratensis) grassland. Plant Soil 2016, 405, 257–264. [Google Scholar] [CrossRef]
- Burrows, G.E.; Tyrl, R.J. Toxic Plants of North America, 2nd ed.; Wiley-Blackwell: Oxford, UK, 2012; ISBN 9781118413425. [Google Scholar]
- Schimel, J.P.; Van Cleve, K.; Cates, R.G.; Clausen, T.P.; Reichardt, P.B. Effects of balsam poplar (Populus balsamifera) tannins and low molecular weight phenolics on microbial activity in taiga floodplain soil: Implications for changes in N cycling during succession. Can. J. Bot. 1996, 74, 84–90. [Google Scholar] [CrossRef]
- Valachovic, Y.S.; Caldwell, B.A.; Cromack, K., Jr.; Griffiths, R.P. Leaf litter chemistry controls on decomposition of pacific northwest trees and woody shrubs. Can. J. For. Res. 2004, 34, 2131–2147. [Google Scholar] [CrossRef] [Green Version]
- Soto-Barajas, M.C.; Zabalgogeazcoa, I.; Gómez-Fuertes, J.; González-Blanco, V.; Vázquez-de-Aldana, B.R. Epichloë endophytes affect the nutrient and fiber content of Lolium perenne regardless of plant genotype. Plant Soil 2016, 405, 265–277. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Dash, C.K.; Akutse, K.S.; Keppanan, R.; Wang, L. Fungal endophytes: Beyond herbivore management. Front. Microbiol. 2018, 9, 544. [Google Scholar] [CrossRef] [Green Version]
- Bailey, J.K.; Deckert, R.; Schweitzer, J.A.; Rehill, B.J.; Lindroth, R.L.; Gehring, C.; Whitham, T.G. Host plant genetics affect hidden ecological players: Links among populus, condensed tannins, and fungal endophyte infection. Can. J. Bot. 2005, 83, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Schweitzer, J.A.; Madritch, M.D.; Bailey, J.K.; LeRoy, C.J.; Fischer, D.G.; Rehill, B.J.; Lindroth, R.L.; Hagerman, A.E.; Wooley, S.C.; Hart, S.C.; et al. From genes to ecosystems: The genetic basis of condensed tannins and their role in nutrient regulation in a populus model system. Ecosystems 2008, 11, 1005–1020. [Google Scholar] [CrossRef]
- Sieber, T.N. Endophytic fungi in forest trees: Are they mutualists? Fungal Biol. Rev. 2007, 21, 75–89. [Google Scholar] [CrossRef]
- Šnajdr, J.; Cajthaml, T.; Valášková, V.; Merhautová, V.; Petránková, M.; Spetz, P.; Leppänen, K.; Baldrian, P. Transformation of Quercus petraea litter: Successive changes in litter chemistry are reflected in differential enzyme activity and changes in the microbial community composition. FEMS Microbiol. Ecol. 2011, 75, 291–303. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Chen, L. The role of endophytic fungal individuals and communities in the decomposition of pinus massoniana needle litter. PLoS ONE 2014, 9, e105911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, R.E.; Schmidt, S.K. Fungal and bacterial responses to phenolic compounds and amino acids in high altitude barren soils. Soil Biol. Biochem. 2002, 34, 989–995. [Google Scholar] [CrossRef]
- Souto, X.C.; Chiapusio, G.; Pellissier, F. Relationships between phenolics and soil microorganisms in spruce forests: Significance for natural regeneration. J. Chem. Ecol. 2000, 26, 2025–2034. [Google Scholar] [CrossRef]
- Faeth, S.H.; Shochat, E. Inherited microbial symbionts increase herbivore abundances and alter arthropod diversity on a native grass. Ecology 2010, 91, 1329–1343. [Google Scholar] [CrossRef]
- Jackrel, S.L.; Wootton, J.T. Cascading effects of induced terrestrial plant defences on aquatic and terrestrial ecosystem function. Proc. Biol. Sci. 2015, 282, 20142522. [Google Scholar] [CrossRef] [Green Version]
- Purahong, W.; Hyde, K.D. Effects of fungal endophytes on grass and non-grass litter decomposition rates. Fungal Divers. 2011, 47, 1–7. [Google Scholar] [CrossRef]
- Saikkonen, K.; Mikola, J.; Helander, M. Endophytic phyllosphere fungi and nutrient cycling in terrestrial ecosystems. Curr. Sci. 2015, 109, 121–126. [Google Scholar]
- Schulz, B.; Boyle, C. The endophytic continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef] [Green Version]
- Matsukura, K.; Hirose, D.; Kagami, M.; Osono, T.; Yamaoka, Y. Geographical distributions of rhytismataceous fungi on Camellia japonica leaf litter in Japan. Fungal Ecol. 2017, 26, 37–44. [Google Scholar] [CrossRef]
- Chauvet, E.; Cornut, J.; Sridhar, K.R.; Selosse, M.-A.; Bärlocher, F. Beyond the water column: Aquatic hyphomycetes outside their preferred habitat. Fungal Ecol. 2016, 19, 112–127. [Google Scholar] [CrossRef] [Green Version]
- Seena, S.; Monroy, S. Preliminary insights into the evolutionary relationships of aquatic hyphomycetes and endophytic fungi. Fungal Ecol. 2016, 19, 128–134. [Google Scholar] [CrossRef]




| Taxa | Associated Host Genus | Fungal Order | Indicator Value | p-Value |
|---|---|---|---|---|
| Rhytisma | Acer | Rhytismatales | 0.816497 | 0.005 |
| Boeremia | Alnus | Pleosporales | 0.912871 | 0.005 |
| Ophiognomonia | Alnus | Diaporthales | 0.5 | 0.015 |
| Pseudopithomyces | Alnus | Pleosporales | 0.5 | 0.03 |
| Amphisphaeriaceae | Fagus | Xylariales | 0.57735 | 0.025 |
| Apiognomonia | Fagus | Diaporthales | 0.57735 | 0.025 |
| Arthrinium | Fagus | Sordariales | 0.5 | 0.015 |
| Ascochyta | Fagus | Pleosporales | 0.745356 | 0.005 |
| Beauveria | Fagus | Hypocreales | 0.57735 | 0.025 |
| Cryptococcus | Fagus | Tremellales | 0.537484 | 0.045 |
| Cylindrium | Fagus | Hypocreales | 0.447214 | 0.05 |
| Discosia | Fagus | Amphisphaeriales | 0.547723 | 0.01 |
| Dothideomycetes | Fagus | NA | 0.471405 | 0.05 |
| Fungal sp. (undetermined) | Fagus | NA | 0.573026 | 0.025 |
| Geniculosporium | Fagus | Xylariales | 0.645497 | 0.005 |
| Hypoxylon | Fagus | Xylariales | 0.456436 | 0.035 |
| Mycosphaerella | Fagus | Capnodiales | 0.707107 | 0.005 |
| Nectriaceae | Fagus | Hypocreales | 0.544331 | 0.025 |
| Pezizomycotina | Fagus | NA | 0.57735 | 0.025 |
| Phaeosphaeria | Fagus | Pleosporales | 0.447214 | 0.04 |
| Phialemoniopsis | Fagus | Cephalothecales | 0.57735 | 0.025 |
| Hormonema | Picea | Dothideales | 0.632456 | 0.005 |
| Lachnum | Picea | Helotiales | 0.489898 | 0.03 |
| Lophodermium | Picea | Rhytismatales | 0.6 | 0.005 |
| Rhizoctonia | Picea | Cantharellales | 0.516398 | 0.02 |
| Thysanophora | Picea | Eurotiales | 0.632456 | 0.005 |
| Rhabdocline | Pseudotsuga | Helotiales | 0.894427 | 0.005 |
| Helminthosporium | Quercus | Pleosporales | 0.516398 | 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolfe, E.R.; Ballhorn, D.J. Do Foliar Endophytes Matter in Litter Decomposition? Microorganisms 2020, 8, 446. https://doi.org/10.3390/microorganisms8030446
Wolfe ER, Ballhorn DJ. Do Foliar Endophytes Matter in Litter Decomposition? Microorganisms. 2020; 8(3):446. https://doi.org/10.3390/microorganisms8030446
Chicago/Turabian StyleWolfe, Emily R., and Daniel J. Ballhorn. 2020. "Do Foliar Endophytes Matter in Litter Decomposition?" Microorganisms 8, no. 3: 446. https://doi.org/10.3390/microorganisms8030446
APA StyleWolfe, E. R., & Ballhorn, D. J. (2020). Do Foliar Endophytes Matter in Litter Decomposition? Microorganisms, 8(3), 446. https://doi.org/10.3390/microorganisms8030446