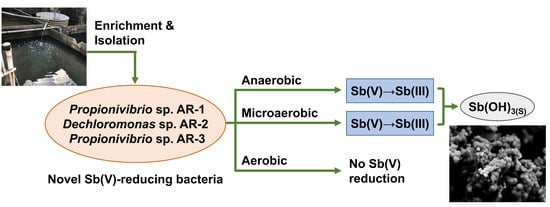
Isolation and Characterization of Facultative-Anaerobic Antimonate-Reducing Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Media and Cultivation Conditions
2.2. Enrichment of the Sb(V)-Reducing Bacteria
2.3. Isolation of Sb(V)-Reducing Bacteria
2.4. Phylogenetic, Physiological and Biochemical Characterization
2.5. Characterization of Growth Ability
2.6. Sb(V) Reduction Experiments
2.7. Analytical Methods
2.8. Solid Analysis
3. Results and Discussion
3.1. Isolation and Identification of Sb(V)-Reducing Bacteria
3.2. Physiological and Biochemical Characteristics of Strains AR-2 and AR-3
3.3. Anaerobic Sb(V) Reduction by Strains AR-2 and AR-3
3.4. Effects of Oxygen on Sb(V) Reduction by Strains AR-2 and AR-3
3.5. Properties of Precipitates Formed during Sb(V) Reduction by Strains AR-2 and AR-3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ungreanu, G.; Santos, S.; Boaventura, R.; Botelho, C. Arsenic and antimony in water and wastewater: Overview of removal techniques with special reference to latest advances in adsorption. J. Environ. Manage. 2015, 15, 326–342. [Google Scholar] [CrossRef]
- Filella, M.; Belzile, N.; Chen, Y.W. Antimony in the environment: A review focused on natural waters I. Occurrence. Earth-Sci. Rev. 2002, 57, 125–176. [Google Scholar] [CrossRef]
- Okkenhaug, G.; Zhu, Y.G.; Luo, L.; Lei, M.; Mulder, J. Distribution, speciation and availability of antimony (Sb) in soils and terrestrial plants from an active Sb mining area. Environ. Pollut. 2011, 159, 2427–2434. [Google Scholar] [CrossRef]
- Filella, M.; Belzile, N.; Chen, Y.W. Antimony in the environment: A review focused on natural waters II. Relevant solution chemistry. Earth-Sci. Rev. 2002, 59, 265–285. [Google Scholar] [CrossRef]
- Scheinost, A.C.; Rossberg, A.; Vantelon, D.; Xifra, I.; Kretzschmar, R.; Leuz, A.; Funke, H.; Johnson, C.A. Quantitative antimony speciation in shooting-range soils by EXAFS spectroscopy. Geochim. Cosmochim. Acta 2006, 70, 3299–3312. [Google Scholar] [CrossRef]
- He, M.; Wang, N.; Long, X.; Zhang, C.; Ma, C.; Zhong, Q.; Wang, A.; Wang, Y.; Pervaiz, A.; Shan, J. Antimony speciation in the environment: Recent advances in understanding the biogeochemical processes and ecological effects. J. Environ. Sci. 2018, 75, 14–39. [Google Scholar] [CrossRef]
- Cutter, G.A.; Cutter, L.S.; Featherstone, A.M.; Lohrenz, S.E. Antimony and arsenic biogeochemistry in the western Atlantic Ocean. Deep-Sea Res. Part II 2001, 48, 2895–2915. [Google Scholar] [CrossRef]
- Fu, Z.; Wu, F.; Amarasiriwardena, D.; Mo, C.; Liu, B.; Zhu, J.; Deng, Q.; Liao, H. Antimony, arsenic and mercury in the aquatic environment and fish in a large antimony mining area in Hunan, China. Sci. Total Environ. 2010, 408, 3403–3410. [Google Scholar] [CrossRef]
- Ritchie, V.J.; Ilgen, A.G.; Mueller, S.H.; Thomas, P.T.; Goldfarb, R.J. Mobility and chemical fate of antimony and arsenic in historic mining environments of the Kantishna Hills district, Denali National Park and Preserve, Alaska. Chem. Geol. 2013, 335, 172–188. [Google Scholar] [CrossRef]
- Sui, Q.; Cao, X.; Lu, S.; Zhao, W.; Qiu, Z.; Yu, G. Occurrence, sources and fate of pharmaceuticals and personal care products in the groundwater: A review. Emerg. Contam. 2015, 1, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zheng, B.; He, Y.; Zhou, Y.; Chen, X.; Ruan, S.; Yang, Y.; Dai, C.; Tang, L. Antimony contamination, consequences and removal techniques: A review. Ecotoxicol. Environ. Saf. 2018, 156, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Q.; Oremland, R.S.; Kulp, T.R.; Rensing, C.; Wang, G. Microbial antimony biogeochemistry: Enzymes, regulation and related metabolic pathways. Appl. Environ. Microbiol. 2016, 82, 5482–5495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulp, T.R.; Miller, L.G.; Braiotta, F.; Webb, S.M.; Kocar, B.D.; Blum, J.S.; Oremland, R.S. Microbiological reduction of Sb(V) in anoxic freshwater sediments. Environ. Sci. Technol. 2014, 48, 218–226. [Google Scholar] [CrossRef]
- Wang, L.; Ye, L.; Yu, Y.; Jing, C. Antimony redox biotransformation in the subsurface: Effect of indigenous Sb(V) respiring microbiota. Environ. Sci. Technol. 2018, 52, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wu, M.; Gao, N.; Chu, W.; An, N.; Wang, Q.; Wang, S. Removal of antimonate from wastewater by dissimilatory bacterial reduction: Role of the coexisting sulfate. J. Hazard. Mater. 2018, 341, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Abin, C.A.; Hollibaugh, T.J. Dissimilatory antimonate reduction and production of antimony trioxide microcrystals by a novel microorganism. Environ. Sci. Technol. 2014, 48, 681–688. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Lee, J. Isolation and characterization of antimony-reducing bacteria from sediments collected in the vicinity of an antimony factory. Geomicrobiol. J. 2014, 31, 855–861. [Google Scholar] [CrossRef]
- Macy, J.M.; Thomas, A.M.; Donald, G.K. Selenate reduction by a Pseudomonas species: A new mode of anaerobic respiration. FEMS Microbiol. Lett. 1989, 61, 195–198. [Google Scholar] [CrossRef]
- Oremland, R.S.; Blum, J.S.; Culbertson, C.W.; Visscher, P.T.; Miller, L.G.; Dowdle, P.; Strohmaier, F.E. Isolation, growth, and metabolism of an obligately anaerobic, selenite-respiring bacterium, strain SES-3. Appl. Environ. Microbiol. 1994, 60, 3011–3019. [Google Scholar] [CrossRef] [Green Version]
- Somerville, G.A.; Proctor, R.A. Cultivation conditions and the diffusion of oxygen into culture media: The rationale for the flask-to-medium ratio in microbiology. BMC Microbiol. 2013, 13, 9. [Google Scholar] [CrossRef] [Green Version]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S Ribosomal DNA Amplification for Phylogenetic Study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, D.J.; Pace, B.; Olsen, G.J.; Stahi, D.A.; Sogin, M.L.; Pace, N.R. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc. Natl. Acad. Sci. USA 1985, 82, 6955–6959. [Google Scholar] [CrossRef] [Green Version]
- Achenbach, L.A.; Michaelidou, U.; Bruce, R.A.; Coates, J.D.; Fryman, J. Dechloromonas agitata gen. nov., sp. nov. and Dechlorosoma suillum gen. nov., sp. nov., two novel environmentally dominant (per)chlorate-reducing bacteria and their phylogenetic position. Int. J. Syst. Evol. Microbiol. 2001, 51, 527–533. [Google Scholar] [CrossRef]
- Thrash, J.C.; Pollock, J.; Torok, T.; Coates, J.D. Description of the novel perchlorate-reducing bacteria Dechlorobacter hydrogenophilus gen. nov., sp. nov. and Propionivibrio militaris, sp. nov. Appl. Microbiol. Biotechnol. 2010, 86, 335–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salineo, K.K.; Keller, K.; Feil, W.S.; Feil, H.; Trong, S.; Bartolo, G.D.; Lapidus, A. Metabolic analysis of the soil microbe Dechloromonas aromatica str. RCB: Indications of a surprisingly complex life-style and cryptic anaerobic pathways for aromatic degradation. BMC Genom. 2009, 10, 351. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.-Y.; Dong, Q.-Y.; Zhao, H.-P. Oxygen exposure deprives antimonate-reducing capability of a methane fed biofilm. Sci. Total Environ. 2018, 644, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-Y.; Dong, Q.-Y.; Rittman, B.E.; Zhao, H.-P. Bioreduction of antimonate by anaerobic methane oxidation in a membrane biofilm batch reactor. Environ. Sci. Technol. 2018, 52, 8693–8700. [Google Scholar] [CrossRef]
- Zotov, A.V.; Shikina, N.D.; Akinfiev, N.N. Thermodynamic properties of the Sb(III) hydroxide complex Sb(OH)3(aq) at hydrothermal conditions. Geochimica et Cosmochimica Acta 2003, 67, 1821–1836. [Google Scholar] [CrossRef]
- Lai, C.Y.; Wen, L.L.; Zhang, Y.; Luo, S.S.; Wang, Q.Y.; Luo, Y.H.; Chen, R.; Yang, X.; Rittmann, B.E.; Zhao, H.P. Autotrophic antimonate bio-reduction using hydrogen as the electron donor. Water Res. 2016, 88, 467–474. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Park, Y.; Lee, T. Microbial antimonate reduction with a solid-state electrode as the sole electron donor: A novel approach for antimony bioremediation. J. Hazard. Mater. 2019, 377, 179–185. [Google Scholar] [CrossRef]



| Strain | AR-2 | AR-3 |
|---|---|---|
| Cellular morphology | Rod (0.7–0.8 × 1.0–2.0 μm) | Rod (0.5–0.7 × 1.5–3.0 μm) |
| Gram stain | − | − |
| Spore | − | − |
| Motility | + | + |
| Catalase test | + | + |
| Oxidase test | + | + |
| O/F test | − | − |
| Growable temperature in LB medium | 15–37 °C | 15–37 °C |
| Growable pH in LB medium | 6–8.5 | 5–8.5 |
| Closest relative of 16S rRNA gene sequence | Dechloromonas agitata CKBT (Similarity: 98.4%) | Propionivibrio militaris MPT (Similarity: 99.8%) |
| Strain | Elemental Distribution in Precipitates (%) | ||
|---|---|---|---|
| O | S | Sb | |
| AR-2 | 78.2 | nd | 21.8 |
| AR-3 | 82.4 | 0.1 | 17.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Hosokawa, H.; Sadakane, T.; Kuroda, M.; Inoue, D.; Nishikawa, H.; Ike, M. Isolation and Characterization of Facultative-Anaerobic Antimonate-Reducing Bacteria. Microorganisms 2020, 8, 1435. https://doi.org/10.3390/microorganisms8091435
Yang Z, Hosokawa H, Sadakane T, Kuroda M, Inoue D, Nishikawa H, Ike M. Isolation and Characterization of Facultative-Anaerobic Antimonate-Reducing Bacteria. Microorganisms. 2020; 8(9):1435. https://doi.org/10.3390/microorganisms8091435
Chicago/Turabian StyleYang, Ziran, Hisaaki Hosokawa, Takuya Sadakane, Masashi Kuroda, Daisuke Inoue, Hiroshi Nishikawa, and Michihiko Ike. 2020. "Isolation and Characterization of Facultative-Anaerobic Antimonate-Reducing Bacteria" Microorganisms 8, no. 9: 1435. https://doi.org/10.3390/microorganisms8091435
APA StyleYang, Z., Hosokawa, H., Sadakane, T., Kuroda, M., Inoue, D., Nishikawa, H., & Ike, M. (2020). Isolation and Characterization of Facultative-Anaerobic Antimonate-Reducing Bacteria. Microorganisms, 8(9), 1435. https://doi.org/10.3390/microorganisms8091435