Artificial Diets Modulate Infection Rates by Nosema ceranae in Bumblebees
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bees
2.2. Parasites
2.3. Infections
2.4. Artificial Diets
2.5. Experiment 1. Does Diet Influence N. ceranae Infection?
2.5.1. Survival
2.5.2. Parasite Prevalence
2.5.3. Parasite Loads
2.6. Experiment 2. Do Bumblebees Evacuate N. ceranae Spores through Faeces?
2.7. Experiment 3. Does N. ceranae Invade Bumblebee Gut Cells?
2.8. Statistical Analyses
3. Results
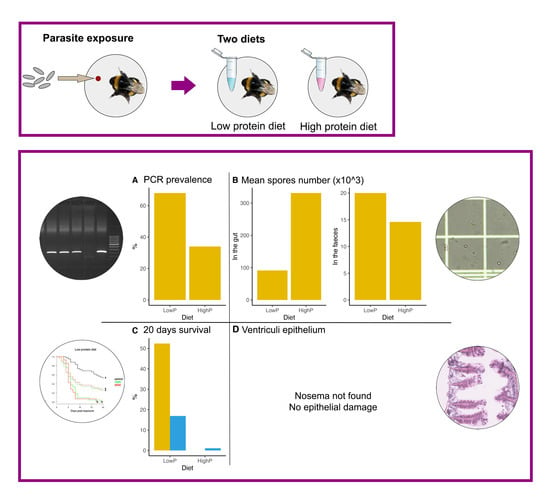
3.1. Experiment 1. Does Diet Influence N. ceranae Infection?
3.1.1. Parasite Prevalence
3.1.2. Parasite Loads
3.1.3. Survival
3.2. Experiment 2. Do Bumblebees Evacuate N. ceranae Spores through Faeces?
3.3. Experiment 3. Does N. ceranae Invade Bumblebee Gut Cells?
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmid-Hempel, P. On the evolutionary ecology of host-parasite interactions: Addressing the question with regard to bumblebees and their parasites. Naturwissenschaften 2001, 88, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernández, R.; Bartolomé, C.; Soroker, V.; Higes, M.; Chejanovsky, N.; Le Conte, Y.; Dalmon, A.; Dussaubat, C.; García-Palencia, P.; Meana, A.; et al. Nosema ceranae in Apis mellifera: A 12 years post detection perspective. Environ. Microbiol. 2018, 20, 1302–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hristov, P.; Shumkova, R.; Palova, N.; Neov, B. Factors Associated with Honey Bee Colony Losses: A Mini-Review. Vet. Sci. 2020, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Moracho, T.; Heeb, P.; Lihoreau, M. Effects of parasites and pathogens on bee cognition. Ecol. Èntomol. 2017, 42, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Klein, S.; Cabirol, A.; Devaud, J.-M.; Barron, A.B.; Lihoreau, M. Why Bees Are So Vulnerable to Environmental Stressors. Trends Ecol. Evol. 2017, 32, 268–278. [Google Scholar] [CrossRef]
- Higes, M.; García-Palencia, P.; Martín-Hernández, R.; Meana, A. Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J. Invertebr. Pathol. 2007, 94, 211–217. [Google Scholar] [CrossRef]
- García-Palencia, P.; Martín-Hernández, R.; González-Porto, A.-V.; Marin, P.; Meana, A.; Higes, M. Natural infection by Nosema ceranae causes similar lesions as in experimentally infected caged-worker honey bees (Apis mellifera). J. Apic. Res. 2010, 49, 278–283. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invertebr. Pathol. 2009, 100, 185–188. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Copley, T.; Jabaji, S. Gas chromatography–mass spectrometry metabolite profiling of worker honey bee (Apis mellifera L.) hemolymph for the study of Nosema ceranae infection. J. Insect Physiol. 2012, 58, 1349–1359. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Cook, S.C. Chronic Nosema ceranae infection inflicts comprehensive and persistent immunosuppression and accelerated lipid loss in host Apis mellifera honey bees. Int. J. Parasitol. 2018, 48, 433–444. [Google Scholar] [CrossRef]
- Aufauvre, J.; Biron, D.G.; Vidau, C.; Fontbonne, R.; Roudel, M.; Diogon, M.; Viguès, B.; Belzunces, L.P.; Delbac, F.; Blot, N. Parasite-insecticide interactions: A case study of Nosema ceranae and fipronil synergy on honeybee. Sci. Rep. 2012, 2, 326. [Google Scholar] [CrossRef] [PubMed]
- Alaux, C.; Brunet, J.-L.; Dussaubat, C.; Mondet, F.; Tchamitchian, S.; Cousin, M.; Brillard, J.; Baldy, A.; Belzunces, L.P.; Le Conte, Y. Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environ. Microbiol. 2010, 12, 774–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-Hernández, R.; Botías, C.; Barrios, L.; Martínez-Salvador, A.; Meana, A.; Mayack, C.; Higes, M. Comparison of the energetic stress associated with experimental Nosema ceranae and Nosema apis infection of honeybees (Apis mellifera). Parasitol. Res. 2011, 109, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Holt, H.L.; Aronstein, K.; Grozinger, C.M. Chronic parasitization by Nosema microsporidia causes global expression changes in core nutritional, metabolic and behavioral pathways in honey bee workers (Apis mellifera). BMC Genom. 2013, 14, 799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-Hernández, R.; Higes, M.; Sagastume, S.; Juarranz, Á.; Dias-Almeida, J.; Budge, G.E.; Meana, A.; Boonham, N. Microsporidia infection impacts the host cell’s cycle and reduces host cell apoptosis. PLoS ONE 2017, 12, e0170183. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Martín-Hernández, R.; Garrido-Bailón, E.; González-Porto, A.V.; García-Palencia, P.; Meana, A.; Del Nozal, M.J.; Mayo, R.; Bernal, J.L. Honeybee colony collapse due to Nosema ceranaein professional apiaries. Environ. Microbiol. Rep. 2009, 1, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Goblirsch, M.; Huang, Z.Y.; Spivak, M. Physiological and Behavioral Changes in Honey Bees (Apis mellifera) Induced by Nosema ceranae Infection. PLoS ONE 2013, 8, e58165. [Google Scholar] [CrossRef]
- Perry, C.J.; Søvik, E.; Myerscough, M.R.; Barron, A.B. Rapid behavioral maturation accelerates failure of stressed honey bee colonies. Proc. Natl. Acad. Sci. USA 2015, 112, 3427–3432. [Google Scholar] [CrossRef] [Green Version]
- Kralj, J.; Fuchs, S. Nosema sp. influences flight behavior of infected honey bee (Apis mellifera) foragers. Apidologie 2009, 41, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Dosselli, R.; Grassl, J.; Carson, A.; Simmons, L.W.; Baer, B. Flight behaviour of honey bee (Apis mellifera) workers is altered by initial infections of the fungal parasite Nosema apis. Sci. Rep. 2016, 6, 36649. [Google Scholar] [CrossRef] [Green Version]
- Dussaubat, C.; Maisonnasse, A.; Crauser, D.; Beslay, D.; Costagliola, G.; Soubeyrand, S.; Kretzchmar, A.; Le Conte, Y. Flight behavior and pheromone changes associated to Nosema ceranae infection of honey bee workers (Apis mellifera) in field conditions. J. Invertebr. Pathol. 2013, 113, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; McMahon, D.P.; Lim, K.S.; Pull, C.D.; Clark, S.J.; Paxton, R.J.; Osborne, J.L. So Near and Yet So Far: Harmonic Radar Reveals Reduced Homing Ability of Nosema Infected Honeybees. PLoS ONE 2014, 9, e103989. [Google Scholar] [CrossRef] [Green Version]
- Piiroinen, S.; Goulson, D. Chronic neonicotinoid pesticide exposure and parasite stress differentially affects learning in honeybees and bumblebees. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gage, S.L.; Kramer, C.; Calle, S.; Carroll, M.; Heien, M.; DeGrandi-Hoffman, G. Nosema ceranae parasitism impacts olfactory learning and memory and neurochemistry in honey bees (Apis mellifera). J. Exp. Biol. 2017, 221, jeb161489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plischuk, S.; Martín-Hernández, R.; Prieto, L.; Lucía, M.; Botías, C.; Meana, A.; Abrahamovich, A.H.; Lange, C.; Higes, M. South American native bumblebees (Hymenoptera: Apidae) infected by Nosema ceranae (Microsporidia), an emerging pathogen of honeybees (Apis mellifera). Environ. Microbiol. Rep. 2009, 1, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, W.; Wu, J.; Peng, W.; An, J.; Schmid-Hempel, P.; Schmid-Hempel, R. Diversity of Nosema associated with bumblebees (Bombus spp.) from China. Int. J. Parasitol. 2012, 42, 49–61. [Google Scholar] [CrossRef]
- Graystock, P.; Yates, K.; Darvill, B.; Goulson, D.; Hughes, W.O.H. Emerging dangers: Deadly effects of an emergent parasite in a new pollinator host. J. Invertebr. Pathol. 2013, 114, 114–119. [Google Scholar] [CrossRef]
- Porrini, M.P.; Porrini, L.P.; Garrido, P.M.; de Melo e Silva Neto, C.; Porrini, D.P.; Muller, F.; Nuñez, L.A.; Alvarez, L.; Iriarte, P.F.; Eguaras, M.J. Nosema ceranae in South American Native Stingless Bees and Social Wasp. Microb. Ecol. 2017, 74, 761–764. [Google Scholar] [CrossRef]
- Purkiss, T.; Lach, L. Pathogen spillover from Apis mellifera to a stingless bee. Proc. R. Soc. B Biol. Sci. 2019, 286, 20191071. [Google Scholar] [CrossRef] [Green Version]
- Malysh, J.M.; Ignatieva, A.N.; Artokhin, K.S.; Frolov, A.N.; Tokarev, Y.S. Natural infection of the beet webworm Loxostege sticticalis L. (Lepidoptera: Crambidae) with three Microsporidia and host switching in Nosema ceranae. Parasitol. Res. 2018, 117, 3039–3044. [Google Scholar] [CrossRef]
- Michener, C.D. The Bees of the World, 2nd ed.; Johns Hopkins University Press: Baltimore, MA, USA, 2007; ISBN 978-0-8018-8573-0. [Google Scholar]
- Cremer, S.; Armitage, S.A.O.; Schmid-Hempel, P. Social immunity. Curr. Biol. 2007, 17, R693–R702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piiroinen, S.; Botías, C.; Nicholls, E.; Goulson, D. No effect of low-level chronic neonicotinoid exposure on bumblebee learning and fecundity. PeerJ 2016, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gisder, S.; Horchler, L.; Pieper, F.; Schüler, V.; Šima, P.; Genersch, E. Rapid Gastrointestinal Passage May Protect Bombus terrestris from Becoming a True Host for Nosema ceranae. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef] [PubMed]
- Botías, C.; Jones, J.C.; Pamminger, T.; Bartomeus, I.; Hughes, W.O.H.; Goulson, D. Multiple stressors interact to impair the performance of bumblebee (Bombus terrestris) colonies. J. Anim. Ecol. 2020, 1365–2656. [Google Scholar] [CrossRef]
- Roberts, K.E.; Hughes, W.O.H. Horizontal transmission of a parasite is influenced by infected host phenotype and density. Parasitology 2015, 142, 395–405. [Google Scholar] [CrossRef]
- Rutrecht, S.T.; Klee, J.; Brown, M.J.F. Horizontal transmission success of Nosema bombi to its adult bumble bee hosts: Effects of dosage, spore source and host age. Parasitology 2007, 134, 1719–1726. [Google Scholar] [CrossRef]
- Urbieta-Magro, A.; Higes, M.; Meana, A.; Barrios, L.; Martín-Hernández, R. Age and Method of Inoculation Influence the Infection of Worker Honey Bees (Apis mellifera) by Nosema ceranae. Insects 2019, 10, 417. [Google Scholar] [CrossRef] [Green Version]
- Fürst, M.; McMahon, D.P.; Osborne, J.L.; Paxton, R.J.; Brown, M.J.F. Disease associations between honeybees and bumblebees as a threat to wild pollinators. Nat. Cell Biol. 2014, 506, 364–366. [Google Scholar] [CrossRef]
- Ponton, F.; Wilson, K.; Cotter, S.C.; Raubenheimer, D.; Simpson, S.J. Nutritional Immunology: A Multi-Dimensional Approach. PLoS Pathog. 2011, 7, e1002223. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.P.; Cory, J.S.; Wilson, K.; Raubenheimer, D.; Simpson, S.J. Flexible diet choice offsets protein costs of pathogen resistance in a caterpillar. Proc. R. Soc. B Boil. Sci. 2005, 273, 823–829. [Google Scholar] [CrossRef] [Green Version]
- Povey, S.; Cotter, S.C.; Simpson, S.J.; Wilson, K. Dynamics of macronutrient self-medication and illness-induced anorexia in virally infected insects. J. Anim. Ecol. 2013, 83, 245–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotter, S.C.; Simpson, S.J.; Raubenheimer, D.; Wilson, K. Macronutrient balance mediates trade-offs between immune function and life history traits. Funct. Ecol. 2010, 25, 186–198. [Google Scholar] [CrossRef]
- Brunner, F.S.; Schmid-Hempel, P.; Barribeau, S.M. Protein-poor diet reduces host-specific immune gene expression in Bombus terrestris. Proc. R. Soc. B Boil. Sci. 2014, 281, 20140128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinderer, T.E.; Elliott, K.D. Worker Honey Bee Response to Infection with Nosema apis: Influence of Diet. J. Econ. Entomol. 1977, 70, 431–433. [Google Scholar] [CrossRef]
- Jack, C.J.; Uppala, S.S.; Lucas, H.M.; Sagili, R.R. Effects of pollen dilution on infection of Nosema ceranae in honey bees. J. Insect Physiol. 2016, 87, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Tritschler, M.; Vollmann, J.J.; Yañez, O.; Chejanovsky, N.; Crailsheim, K.; Neumann, P. Protein nutrition governs within-host race of honey bee pathogens. Sci. Rep. 2017, 7, 14988. [Google Scholar] [CrossRef] [Green Version]
- Porrini, M.P.; Sarlo, E.G.; Medici, S.K.; Garrido, P.M.; Porrini, D.P.; Damiani, N.; Eguaras, M.J. Nosema ceranae development in Apis mellifera: Influence of diet and infective inoculum. J. Apic. Res. 2011, 50, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Mason, P.A.; Smilanich, A.M.; Singer, M.S. Reduced consumption of protein-rich foods follows immune challenge in a polyphagous caterpillar. J. Exp. Biol. 2014, 217, 2250–2260. [Google Scholar] [CrossRef] [Green Version]
- Poissonnier, L.-A.; Lihoreau, M.; Gomez-Moracho, T.; Dussutour, A.; Buhl, J. A theoretical exploration of dietary collective medication in social insects. J. Insect Physiol. 2018, 106, 78–87. [Google Scholar] [CrossRef]
- Higes, M.; García-Palencia, P.; Urbieta, A.; Nanetti, A.; Martín-Hernández, R. Nosema apis and Nosema ceranae Tissue Tropism in Worker Honey Bees (Apis mellifera). Vet. Pathol. 2019, 57, 132–138. [Google Scholar] [CrossRef]
- Martín-Hernández, R.; Meana, A.; Prieto, L.; Salvador, A.M.; Garrido-Bailón, E.; Higes, M. Outcome of Colonization of Apis mellifera by Nosema ceranae. Appl. Environ. Microbiol. 2007, 73, 6331–6338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klee, J.; Tay, W.T.; Paxton, R.J. Specific and sensitive detection of Nosema bombi (Microsporidia: Nosematidae) in bumble bees (Bombus spp.; Hymenoptera: Apidae) by PCR of partial rRNA gene sequences. J. Invertebr. Pathol. 2006, 91, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Hempel, R.; Tognazzo, M. Molecular Divergence Defines Two Distinct Lineages of Crithidia bombi (Trypanosomatidae), Parasites of Bumblebees. J. Eukaryot. Microbiol. 2010, 57, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Fries, I.; Martin, R.; Meana, A.; García-Palencia, P.; Higes, M. Natural infections of Nosema ceranae in European honey bees. J. Apic. Res. 2006, 47, 230–233. [Google Scholar] [CrossRef]
- Fries, I.; Chauzat, M.-P.; Chen, Y.-P.; Doublet, V.; Genersch, E.; Gisder, S.; Higes, M.; McMahon, D.P.; Martín-Hernández, R.; Natsopoulou, M.; et al. Standard methods for Nosema research. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Cantwell, G. Standard methods for counting Nosema spores. Am. Bee J. 1970, 110, 222–223. [Google Scholar]
- Bailey, L. The Epidemiology and Control of Nosema Disease of the Honey-bee. Ann. Appl. Biol. 1955, 43, 379–389. [Google Scholar] [CrossRef]
- Otterstatter, M.C.; Thomson, J.D. Contact networks and transmission of an intestinal pathogen in bumble bee (Bombus impatiens) colonies. Oecologia 2007, 154, 411–421. [Google Scholar] [CrossRef]
- Dussaubat, C.; Sagastume, S.; Gómez-Moracho, T.; Botías, C.; García-Palencia, P.; Martín-Hernández, R.; Le Conte, Y.; Higes, M. Comparative study of Nosema ceranae (Microsporidia) isolates from two different geographic origins. Vet. Microbiol. 2013, 162, 670–678. [Google Scholar] [CrossRef]
- Kafadar, K.; Koehler, J.R.; Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S-Plus, 4th ed.; Springer: New York, NY, USA, 2002; Volume 53, ISBN 038795457. [Google Scholar]
- Akaike, H. Prediction and Entropy. In A Celebration of Statistics; Springer Science and Business Media LLC: New York, NY, USA, 1985; pp. 1–24. [Google Scholar]
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000; ISBN 0387987843. [Google Scholar]
- Vaudo, A.D.; Patch, H.M.; Mortensen, D.A.; Tooker, J.F.; Grozinger, C.M. Macronutrient ratios in pollen shape bumble bee (Bombus impatiens) foraging strategies and floral preferences. Proc. Natl. Acad. Sci. USA 2016, 113, E4035–E4042. [Google Scholar] [CrossRef] [Green Version]
- Kraus, S.; Gómez-Moracho, T.; Pasquaretta, C.; Latil, G.; Dussutour, A.; Lihoreau, M. Bumblebees adjust protein and lipid collection rules to the presence of brood. Curr. Zool. 2019, 65, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Ruedenauer, F.A.; Raubenheimer, D.; Kessner-Beierlein, D.; Grund-Mueller, N.; Noack, L.; Spaethe, J.; Leonhardt, S.D. Best be(e) on low fat: Linking nutrient perception, regulation and fitness. Ecol. Lett. 2020, 23, 545–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.-L.; Alaux, C. Influence of Pollen Nutrition on Honey Bee Health: Do Pollen Quality and Diversity Matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basualdo, M.; Barragán, S.; Antúnez, K. Bee bread increases honeybee haemolymph protein and promote better survival despite of causing higher Nosema ceranae abundance in honeybees. Environ. Microbiol. Rep. 2014, 6, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Mohr, K.I.; Tebbe, C.C. Diversity and phylotype consistency of bacteria in the guts of three bee species (Apoidea) at an oilseed rape field. Environ. Microbiol. 2006, 8, 258–272. [Google Scholar] [CrossRef]
- Wittner, M.; Weiss, L. The Microsporidia and Microsporidiosis; Weiss, L.M., Wittner, M., Eds.; American Society of Microbiology: Washington, DC, USA, 1999; ISBN 9781555811471. [Google Scholar]
- Fleming, J.C.; Schmehl, D.R.; Ellis, J.D. Characterizing the Impact of Commercial Pollen Substitute Diets on the Level of Nosema spp. in Honey Bees (Apis mellifera L.). PLoS ONE 2015, 10, e0132014. [Google Scholar] [CrossRef] [Green Version]
- Otti, O.; Schmid-Hempel, P. Nosema bombi: A pollinator parasite with detrimental fitness effects. J. Invertebr. Pathol. 2007, 96, 118–124. [Google Scholar] [CrossRef]
- Trillo, A.; Brown, M.J.F.; Vilà, M. Prevalence of Nosema microsporidians in commercial bumblebees (Bombus terrestris) is not related to the intensity of their use at the landscape scale. Apidologie 2019, 50, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Bunning, H.; Bassett, L.; Clowser, C.; Rapkin, J.; Jensen, K.; House, C.M.; Archer, C.R.; Hunt, J. Dietary choice for a balanced nutrient intake increases the mean and reduces the variance in the reproductive performance of male and female cockroaches. Ecol. Evol. 2016, 6, 4711–4730. [Google Scholar] [CrossRef] [Green Version]
- Bunning, H.; Rapkin, J.; Belcher, L.; Archer, C.R.; Jensen, K.; Hunt, J. Protein and carbohydrate intake influence sperm number and fertility in male cockroaches, but not sperm viability. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142144. [Google Scholar] [CrossRef]
- Rapkin, J.; Jensen, K.; Archer, C.R.; House, C.M.; Sakaluk, S.K.; Del Castillo, E.; Hunt, J. The Geometry of Nutrient Space–Based Life-History Trade-Offs: Sex-Specific Effects of Macronutrient Intake on the Trade-Off between Encapsulation Ability and Reproductive Effort in Decorated Crickets. Am. Nat. 2018, 191, 452–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.P.; Simpson, S.J.; Clissold, F.J.; Brooks, R.; Ballard, J.W.O.; Taylor, P.W.; Soran, N.; Raubenheimer, D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. USA 2008, 105, 2498–2503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddiex, A.J.; Gosden, T.P.; Bonduriansky, R.; Chenoweth, S.F. Sex-Specific Fitness Consequences of Nutrient Intake and the Evolvability of Diet Preferences. Am. Nat. 2013, 182, 91–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, K.; McClure, C.; Priest, N.K.; Hunt, J. Sex-specific effects of protein and carbohydrate intake on reproduction but not lifespan in Drosophila melanogaster. Aging Cell 2015, 14, 605–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semaniuk, U.; Feden’Ko, K.; Yurkevych, I.S.; Storey, K.B.; Simpson, S.J.; Lushchak, O. Within-diet variation in rates of macronutrient consumption and reproduction does not accompany changes in lifespan in Drosophila melanogaster. Entomol. Exp. Appl. 2018, 166, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Fanson, B.G.; Weldon, C.W.; Pérez-Staples, D.; Simpson, S.J.; Taylor, P.W. Nutrients, not caloric restriction, extend lifespan in Queensland fruit flies (Bactrocera tryoni). Aging Cell 2009, 8, 514–523. [Google Scholar] [CrossRef]
- Maklakov, A.A.; Simpson, S.J.; Zajitschek, F.; Hall, M.D.; Dessmann, J.; Clissold, F.; Raubenheimer, D.; Bonduriansky, R.; Brooks, R.C. Sex-Specific Fitness Effects of Nutrient Intake on Reproduction and Lifespan. Curr. Biol. 2008, 18, 1062–1066. [Google Scholar] [CrossRef] [Green Version]
- Ponton, F.; Wilson, K.; Holmes, A.; Raubenheimer, D.; Robinson, K.L.; Simpson, S.J. Macronutrients mediate the functional relationship between Drosophila and Wolbachia. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142029. [Google Scholar] [CrossRef] [Green Version]
- Altaye, S.Z.; Pirk, C.W.W.; Crewe, R.M.; Nicolson, S.W. Convergence of carbohydrate-biased intake targets in caged worker honeybees fed different protein sources. J. Exp. Biol. 2010, 213, 3311–3318. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.-Q.; Lin, Z.-G.; Huang, S.-K.; Sohr, A.; Wu, L.; Chen, Y.P. Spore Loads May Not be Used Alone as a Direct Indicator of the Severity of Nosema ceranae Infection in Honey Bees Apis mellifera (Hymenoptera: Apidae). J. Econ. Entomol. 2014, 107, 2037–2044. [Google Scholar] [CrossRef]
- Van Der Zee, R.; Gómez-Moracho, T.; Pisa, L.; Sagastume, S.; García-Palencia, P.; Maside, X.; Bartolomé, C.; Martín-Hernández, R.; Higes, M. Virulence and polar tube protein genetic diversity of Nosema ceranae (Microsporidia) field isolates from Northern and Southern Europe in honeybees (Apis mellifera iberiensis). Environ. Microbiol. Rep. 2014, 6, 401–413. [Google Scholar] [CrossRef] [PubMed]



| Spores per Bumblebee | Starvation Duration | Age of Bumblebee | Parasite Exposure | Experimental Condition | Rate of Infection (Based on PCR) | Study |
|---|---|---|---|---|---|---|
| 120,000 | 4 h | Unknown | Micropipette 30% sugar-water | Full colony; first 2 weeks: 50% sucrose solution + pollen 25 °C, 50–60% RH Later on: field | 66% | [35] |
| 6500 | 8 h | Unknown | Hand-fed; 40% sucrose | Groups of 10 40% sucrose solution N/A | 62% | [27] |
| 100,000 | 30–60 min | 2 days old | Individually in a Petri dish 50% sucrose solution | Individually 50% sucrose solution + artificial pollen N/A | 34% No effect on survival | [39] |
| 50,000 | 4 h | Newly hatched | Fed individually Sucrose/pollen solution | Groups of 10–25; 50% (w/v) sucrose + 15% (w/v) pollen 33 °C and 55% RH | 6.13% | [34] |
| 180,000 | 2 h | Unknown | Inoculated individually Sucrose (30%) | Groups of 10; 50% sucrose solution + artificial pollen 25 °C and 50% RH | 3% | [23] |
| 6500–5,000,000 | 4 h | Newly hatched, 4 weeks old or mixed | Fed individually Sucrose-/pollen solution | Groups of 20; 50% (w/v) sucrose + 15% (w/v) pollen 33 °C and 55% RH | 0% | [34] |
| 130,000 | 2 h | Unknown | Micropipette Sucrose (30%) | Microcolonies of 10; 60% sucrose solution + artificial pollen 26 °C, 55% RH | 0% | [33] |
| Diet | Spore Dosage | Exposed | PCR-Positive | Showing Spores |
|---|---|---|---|---|
| Low-protein | 150 K | 44 | 23 (52.27%) | 7 (30.4%) |
| 300 K | 45 | 31 (68.88%) | 9 (29.03%) | |
| High-protein | 150 K | 47 | 14 (29.78%) | 4 (28.5%) |
| 300 K | 46 | 16 (34.78%) | 6 (37.5%) | |
| Total | 182 | 84 (46.15%) | 26 (30.9%) |
| Diet | Spore Dosage | Estimate | SE | z | p |
|---|---|---|---|---|---|
| High-protein | 150 K | 0.2766 | 0.2115 | 1.308 | 0.191 |
| 300 K | 0.1440 | 0.2151 | 0.669 | 0.503 | |
| Low-protein | 150 K | 1.2595 | 0.2792 | 4.511 | <0.001 |
| 300 K | 1.1021 | 0.2808 | 3.925 | <0.001 |
| Days Post-Exposure | Diet | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 5 | 7 | 9 | 11 | 13 | 15 | 17 | 19 | 21 | ||
| Exposed | 6(4) | 14(5) | 14(0) | 12(0) | 11(0) | 9(0) | 11(0) | 6(1) | 6(0) | 4(3) | 2(0) | Low P |
| Control | 2 | 0 | 0 | 0 | 0 | 0 | 13(1) | 0 | 8 | 7 | 7 | |
| Exposed | 8(8) | 14(3) | 15(2) | 12(1) | 12(0) | 9(0) | 8(0) | 8(0) | 7(0) | 5(0) | 0 | High P |
| Control | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Moracho, T.; Durand, T.; Pasquaretta, C.; Heeb, P.; Lihoreau, M. Artificial Diets Modulate Infection Rates by Nosema ceranae in Bumblebees. Microorganisms 2021, 9, 158. https://doi.org/10.3390/microorganisms9010158
Gómez-Moracho T, Durand T, Pasquaretta C, Heeb P, Lihoreau M. Artificial Diets Modulate Infection Rates by Nosema ceranae in Bumblebees. Microorganisms. 2021; 9(1):158. https://doi.org/10.3390/microorganisms9010158
Chicago/Turabian StyleGómez-Moracho, Tamara, Tristan Durand, Cristian Pasquaretta, Philipp Heeb, and Mathieu Lihoreau. 2021. "Artificial Diets Modulate Infection Rates by Nosema ceranae in Bumblebees" Microorganisms 9, no. 1: 158. https://doi.org/10.3390/microorganisms9010158
APA StyleGómez-Moracho, T., Durand, T., Pasquaretta, C., Heeb, P., & Lihoreau, M. (2021). Artificial Diets Modulate Infection Rates by Nosema ceranae in Bumblebees. Microorganisms, 9(1), 158. https://doi.org/10.3390/microorganisms9010158