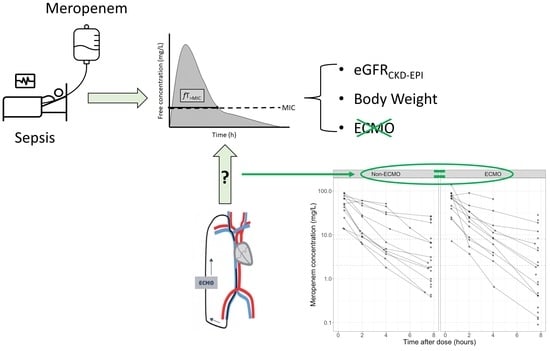
Meropenem Pharmacokinetics and Target Attainment in Critically Ill Patients Are Not Affected by Extracorporeal Membrane Oxygenation: A Matched Cohort Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting, Study Design and Population
2.2. Study Protocol
2.3. Matching Procedure
2.4. PK/PD Target Attainment
2.5. Population PK Modelling
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics and Matching
3.2. PK/PD Target Attainment
3.3. Population Pharmacokinetic Modelling
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Combes, A.; Schmidt, M.; Hodgson, C.L.; Fan, E.; Ferguson, N.D.; Fraser, J.F.; Jaber, S.; Pesenti, A.; Ranieri, M.; Rowan, K.; et al. Extracorporeal life support for adults with acute respiratory distress syndrome. Intensive Care Med. 2020, 46, 2464–2476. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.; Garan, A.R.; Abdelbary, A.; Bacchetta, M.; Bartlett, R.H.; Beck, J.; Belohlavek, J.; Chen, Y.S.; Fan, E.; Ferguson, N.D.; et al. Position paper for the organization of ECMO programs for cardiac failure in adults. Intensive Care Med. 2018, 44, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Karagiannidis, C.; Brodie, D.; Strassmann, S.; Stoelben, E.; Philipp, A.; Bein, T.; Muller, T.; Windisch, W. Extracorporeal membrane oxygenation: Evolving epidemiology and mortality. Intensive Care Med. 2016, 42, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Schellongowski, P.; Patroniti, N.; Taccone, F.S.; Reis Miranda, D.; Reuter, J.; Prodanovic, H.; Pierrot, M.; Dorget, A.; Park, S.; et al. Six-Month Outcome of Immunocompromised Patients with Severe Acute Respiratory Distress Syndrome Rescued by Extracorporeal Membrane Oxygenation. An International Multicenter Retrospective Study. Am. J. Respir. Crit. Care Med. 2018, 197, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Pham, T.; Arcadipane, A.; Agerstrand, C.; Ohshimo, S.; Pellegrino, V.; Vuylsteke, A.; Guervilly, C.; McGuinness, S.; Pierard, S.; et al. Mechanical Ventilation Management during Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome. An International Multicenter Prospective Cohort. Am. J. Respir. Crit. Care Med. 2019, 200, 1002–1012. [Google Scholar] [CrossRef]
- Thiagarajan, R.R.; Barbaro, R.P.; Rycus, P.T.; McMullan, D.M.; Conrad, S.A.; Fortenberry, J.D.; Paden, M.L. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017, 63, 60–67. [Google Scholar] [CrossRef]
- Abrams, D.; Grasselli, G.; Schmidt, M.; Mueller, T.; Brodie, D. ECLS-associated infections in adults: What we know and what we don’t yet know. Intensive Care Med. 2019, 46, 182–191. [Google Scholar] [CrossRef]
- Biffi, S.; Di Bella, S.; Scaravilli, V.; Peri, A.M.; Grasselli, G.; Alagna, L.; Pesenti, A.; Gori, A. Infections during extracorporeal membrane oxygenation: Epidemiology, risk factors, pathogenesis and prevention. Int. J. Antimicrob. Agents 2017, 50, 9–16. [Google Scholar] [CrossRef]
- Grasselli, G.; Scaravilli, V.; Di Bella, S.; Biffi, S.; Bombino, M.; Patroniti, N.; Bisi, L.; Peri, A.M.; Pesenti, A.; Gori, A.; et al. Nosocomial Infections During Extracorporeal Membrane Oxygenation: Incidence, Etiology, and Impact on Patients’ Outcome. Crit. Care Med. 2017, 45, 1726–1733. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Bréchot, N.; Hariri, S.; Guiguet, M.; Luyt, C.E.; Makri, R.; Leprince, P.; Trouillet, J.L.; Pavie, A.; Chastre, J.; et al. Nosocomial infections in adult cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation. Clin. Infect. Dis. 2012, 55, 1633–1641. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.Y.; Ko, W.J.; Tsai, P.R.; Sun, C.C.; Chang, Y.Y.; Lee, C.W.; Chen, Y.C. Infections occurring during extracorporeal membrane oxygenation use in adult patients. J. Thorac. Cardiovasc. Surg. 2010, 140, 1125–1132.e2. [Google Scholar] [CrossRef] [Green Version]
- Dzierba, A.L.; Abrams, D.; Brodie, D. Medicating patients during extracorporeal membrane oxygenation: The evidence is building. Crit. Care 2017, 21, 66. [Google Scholar] [CrossRef] [Green Version]
- Sherwin, J.; Heath, T.; Watt, K. Pharmacokinetics and Dosing of Anti-infective Drugs in Patients on Extracorporeal Membrane Oxygenation: A Review of the Current Literature. Clin. Ther. 2016, 38, 1976–1994. [Google Scholar] [CrossRef] [Green Version]
- Shekar, K.; Roberts, J.A.; McDonald, C.I.; Fisquet, S.; Barnett, A.G.; Mullany, D.V.; Ghassabian, S.; Wallis, S.C.; Fung, Y.L.; Smith, M.T.; et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit. Care 2012, 16, R194. [Google Scholar] [CrossRef] [Green Version]
- Hanberg, P.; Obrink-Hansen, K.; Thorsted, A.; Bue, M.; Tottrup, M.; Friberg, L.E.; Hardlei, T.F.; Soballe, K.; Gjedsted, J. Population Pharmacokinetics of Meropenem in Plasma and Subcutis from Patients on Extracorporeal Membrane Oxygenation Treatment. Antimicrob. Agents Chemother. 2018, 62, e02390-17. [Google Scholar] [CrossRef] [Green Version]
- Donadello, K.; Antonucci, E.; Cristallini, S.; Roberts, J.A.; Beumier, M.; Scolletta, S.; Jacobs, F.; Rondelet, B.; de Backer, D.; Vincent, J.L.; et al. beta-Lactam pharmacokinetics during extracorporeal membrane oxygenation therapy: A case-control study. Int. J. Antimicrob. Agents 2015, 45, 278–282. [Google Scholar] [CrossRef]
- Dhaese, S.A.M.; Farkas, A.; Colin, P.; Lipman, J.; Stove, V.; Verstraete, A.G.; Roberts, J.A.; De Waele, J.J. Population pharmacokinetics and evaluation of the predictive performance of pharmacokinetic models in critically ill patients receiving continuous infusion meropenem: A comparison of eight pharmacokinetic models. J. Antimicrob. Chemother. 2019, 74, 432–441. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Roberts, J.A. Antibiotic dosing during extracorporeal membrane oxygenation: Does the system matter? Curr. Opin. Anaesthesiol. 2020, 33, 71–82. [Google Scholar] [CrossRef]
- Kuhn, D.; Metz, C.; Seiler, F.; Wehrfritz, H.; Roth, S.; Alqudrah, M.; Becker, A.; Bracht, H.; Wagenpfeil, S.; Hoffmann, M.; et al. Antibiotic therapeutic drug monitoring in intensive care patients treated with different modalities of extracorporeal membrane oxygenation (ECMO) and renal replacement therapy: A prospective, observational single-center study. Crit. Care 2020, 24, 664. [Google Scholar] [CrossRef]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.L.; Ramsay, G.; et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 2003, 31, 1250–1256. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013, 39, 165–228. [Google Scholar] [CrossRef]
- Pinder, N.; Brenner, T.; Swoboda, S.; Weigand, M.A.; Hoppe-Tichy, T. Therapeutic drug monitoring of beta-lactam antibiotics—Influence of sample stability on the analysis of piperacillin, meropenem, ceftazidime and flucloxacillin by HPLC-UV. J. Pharm. Biomed. Anal. 2017, 143, 86–93. [Google Scholar] [CrossRef]
- Mortensen, J.S.; Jensen, B.P.; Zhang, M.; Doogue, M. Preanalytical Stability of Piperacillin, Tazobactam, Meropenem, and Ceftazidime in Plasma and Whole Blood Using Liquid Chromatography-Tandem Mass Spectrometry. Ther. Drug Monit. 2019, 41, 538–543. [Google Scholar] [CrossRef]
- Colin, P.; De Bock, L.; T’Jollyn, H.; Boussery, K.; Van Bocxlaer, J. Development and validation of a fast and uniform approach to quantify beta-lactam antibiotics in human plasma by solid phase extraction-liquid chromatography-electrospray-tandem mass spectrometry. Talanta 2013, 103, 285–293. [Google Scholar] [CrossRef]
- Gijsen, M.; Filtjens, B.; Annaert, P.; Armoudjian, Y.; Debaveye, Y.; Wauters, J.; Slaets, P.; Spriet, I. Meropenem Stability in Human Plasma at −20 degrees C: Detailed Assessment of Degradation. Antibiotics 2021, 10, 449. [Google Scholar] [CrossRef]
- Drusano, G.L.; Hutchison, M. The pharmacokinetics of meropenem. Scand. J. Infect. Dis. Suppl. 1995, 96, 11–16. [Google Scholar] [PubMed]
- Burger, R.; Guidi, M.; Calpini, V.; Lamoth, F.; Decosterd, L.; Robatel, C.; Buclin, T.; Csajka, C.; Marchetti, O. Effect of renal clearance and continuous renal replacement therapy on appropriateness of recommended meropenem dosing regimens in critically ill patients with susceptible life-threatening infections. J. Antimicrob. Chemother. 2018, 73, 3413–3422. [Google Scholar] [CrossRef]
- Mattoes, H.M.; Kuti, J.L.; Drusano, G.L.; Nicolau, D.P. Optimizing antimicrobial pharmacodynamics: Dosage strategies for meropenem. Clin. Ther. 2004, 26, 1187–1198. [Google Scholar] [CrossRef]
- Li, C.; Du, X.; Kuti, J.L.; Nicolau, D.P. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob. Agents Chemother. 2007, 51, 1725–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tam, V.H.; Chang, K.T.; Zhou, J.; Ledesma, K.R.; Phe, K.; Gao, S.; Van Bambeke, F.; Sánchez-Díaz, A.M.; Zamorano, L.; Oliver, A.; et al. Determining β-lactam exposure threshold to suppress resistance development in Gram-negative bacteria. J. Antimicrob. Chemother. 2017, 72, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Mouton, J.W.; Vinks, A.A. Continuous infusion of beta-lactams. Curr. Opin. Crit. Care 2007, 13, 598–606. [Google Scholar] [CrossRef]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining antibiotic levels in intensive care unit patients: Are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Dulhunty, J.M.; Bellomo, R.; Lipman, J.; Roberts, J.A. Continuous beta-lactam infusion in critically ill patients: The clinical evidence. Ann. Intensive Care 2012, 2, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. Nlme: Linear and Nonlinear Mixed Effects Models; R Package Version 3; R Core Team: Vienna, Austria, 2014; pp. 1–117. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Park, J.; Shin, D.A.; Lee, S.; Cho, Y.J.; Jheon, S.; Lee, J.C.; Kim, H.C. Investigation of Key Circuit Constituents Affecting Drug Sequestration During Extracorporeal Membrane Oxygenation Treatment. ASAIO J. 2017, 63, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Shekar, K.; Roberts, J.A.; McDonald, C.I.; Ghassabian, S.; Anstey, C.; Wallis, S.C.; Mullany, D.V.; Fung, Y.L.; Fraser, J.F. Protein-bound drugs are prone to sequestration in the extracorporeal membrane oxygenation circuit: Results from an ex vivo study. Crit. Care 2015, 19, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leven, C.; Fillâtre, P.; Petitcollin, A.; Verdier, M.C.; Laurent, J.; Nesseler, N.; Launey, Y.; Tattevin, P.; Bellissant, E.; Flécher, E.; et al. Ex Vivo Model to Decipher the Impact of Extracorporeal Membrane Oxygenation on Beta-lactam Degradation Kinetics. Ther. Drug Monit. 2017, 39, 180–184. [Google Scholar] [CrossRef]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Cheng, D.; Shrivastava, S.; Tzur, D.; Gautam, B.; Hassanali, M. DrugBank: A knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008, 36, D901–D906. [Google Scholar] [CrossRef]
- Shekar, K.; Fraser, J.F.; Taccone, F.S.; Welch, S.; Wallis, S.C.; Mullany, D.V.; Lipman, J.; Roberts, J.A.; Investigators, A.E.S. The combined effects of extracorporeal membrane oxygenation and renal replacement therapy on meropenem pharmacokinetics: A matched cohort study. Crit. Care 2014, 18, 565. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.A.; Lipman, J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 2009, 37, 840–851, quiz 859. [Google Scholar] [CrossRef] [Green Version]
- Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; Paterson, D.L.; et al. Continuous infusion of beta-lactam antibiotics in severe sepsis: A multicenter double-blind, randomized controlled trial. Clin. Infect. Dis. 2013, 56, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Ehmann, L.; Zoller, M.; Minichmayr, I.K.; Scharf, C.; Maier, B.; Schmitt, M.V.; Hartung, N.; Huisinga, W.; Vogeser, M.; Frey, L.; et al. Role of renal function in risk assessment of target non-attainment after standard dosing of meropenem in critically ill patients: A prospective observational study. Crit. Care 2017, 21, 263. [Google Scholar] [CrossRef] [Green Version]
- Gijsen, M.; Wilmer, A.; Meyfroidt, G.; Wauters, J.; Spriet, I. Can augmented renal clearance be detected using estimators of glomerular filtration rate? Crit. Care 2020, 24, 359. [Google Scholar] [CrossRef]


| At Baseline (per Patient) | ECMO (n = 14) | non-ECMO (n = 11) | p-Value |
| Male, n (%) | 9 (64) | 7 (64) | 0.7 |
| Age, median [IQR], years | 47 [39;56] | 68 [63;71] | 0.0008 * |
| Body weight, median [IQR], kg | 84 [54.6;90] | 70 [61;77.5] | 0.337 |
| SOFA score on admission, median [IQR] | 9 [6;12] | 8 [6;11] | 0.62 |
| APACHE II score, median [IQR] | 21 [18;23] | 21 [16;27] | 0.1 |
| ICU mortality, n (%) | 2 (14) | 3 (27) | 0.628 |
| Venoarterial ECMO, n (%) | 2 (14) | NA | NA |
| Dosing interval (on the day of PK sampling) | ECMO (n = 15) | non-ECMO (n = 12) | |
| Days meropenem therapy until measurement, median [IQR] | 2.7 [1.3;5] | 1.5 [1;1.7] | 0.086 |
| Days ECMO therapy until measurement, median [IQR] | 2.6 [1.2;4.4] | NA | NA |
| CRRT, n (%) | 3 (20) | 0 (0) | 0.231 |
| mCrCL24h, median [IQR], mg/dL a | 57 [47;120] | 70 [46;112] | 0.853 |
| eGFRCKD-EPI, median [IQR], mL/min/1.73 m2 b | 106 [80;117] | 97 [92;106] | 0.544 |
| Fluid balance, median [IQR]; mL | 626 [−740;1193] | 568 [−163;976] | 0.942 |
| SOFA score, median [IQR] | 11 [8;14] | 8 [5;9] | 0.006 * |
| Daily meropenem dose, median [IQR] | 3000 [3000;6000] | 3000 [3000;6000] | 0.574 |
| Mechanical ventilation, n (%) | 14 (93) | 9 (75) | 0.294 |
| Vasopressor therapy, n (%) | 9 (60) | 7 (64) | 1 |
| Final Model | (OFV 437.6—CN 336.8) | Bootstrapped Estimate a | ||
|---|---|---|---|---|
| Parameter | Estimate | (%RSE) [Shrinkage] | Median | [95%CI] |
| Fixed effects | ||||
| CL (L/h) | 14.7 | (11) | 14.6 | [11.8–18.1] |
| BWT on CL | 0.75 | FIX | FIX | |
| eGFRCKD-EPI on CL | 1.29 | (16) | 1.31 | [1.03–2.85] |
| Vc (L) | 25.6 | (15) | 24.2 | [16.2–32.9] |
| BWT on Vc | 1 | FIX | FIX | |
| Q (L/h) | 5.51 | (29) | 6.14 | [2.98–37.5] |
| BWT on Q | 0.75 | FIX | FIX | |
| Vp (L) | 8.02 | (23) | 8.75 | [5.54–17.94] |
| BWT on Vp | 1 | FIX | FIX | |
| Random effects | ||||
| IIV on CL (%CV) | 46.8 | (29) [1] | 43.3 | [24.4–68.2] |
| IIV on Vc (%CV) | 61.6 | (22) [8] | 43.3 | [−15.8–68.9] |
| Corr(IIV on CL-Vc) (%CV) | 70.4 | (35) | 62.0 | [28.4–87.1] |
| Residual variability | ||||
| Proportional error (%CV) | 28.8 | (13.3) [15] | 28.2 | [21.2–36.3] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gijsen, M.; Dreesen, E.; Annaert, P.; Nicolai, J.; Debaveye, Y.; Wauters, J.; Spriet, I. Meropenem Pharmacokinetics and Target Attainment in Critically Ill Patients Are Not Affected by Extracorporeal Membrane Oxygenation: A Matched Cohort Analysis. Microorganisms 2021, 9, 1310. https://doi.org/10.3390/microorganisms9061310
Gijsen M, Dreesen E, Annaert P, Nicolai J, Debaveye Y, Wauters J, Spriet I. Meropenem Pharmacokinetics and Target Attainment in Critically Ill Patients Are Not Affected by Extracorporeal Membrane Oxygenation: A Matched Cohort Analysis. Microorganisms. 2021; 9(6):1310. https://doi.org/10.3390/microorganisms9061310
Chicago/Turabian StyleGijsen, Matthias, Erwin Dreesen, Pieter Annaert, Johan Nicolai, Yves Debaveye, Joost Wauters, and Isabel Spriet. 2021. "Meropenem Pharmacokinetics and Target Attainment in Critically Ill Patients Are Not Affected by Extracorporeal Membrane Oxygenation: A Matched Cohort Analysis" Microorganisms 9, no. 6: 1310. https://doi.org/10.3390/microorganisms9061310
APA StyleGijsen, M., Dreesen, E., Annaert, P., Nicolai, J., Debaveye, Y., Wauters, J., & Spriet, I. (2021). Meropenem Pharmacokinetics and Target Attainment in Critically Ill Patients Are Not Affected by Extracorporeal Membrane Oxygenation: A Matched Cohort Analysis. Microorganisms, 9(6), 1310. https://doi.org/10.3390/microorganisms9061310