On the Rarity and Peculiarity of the Early Toarcian (Lower Jurassic) Leukadiellinae Ammonites—Systematic Review and Insights on the Interplay of Environmental Stress, Evolution and Biodiversity †
Abstract
:1. Introduction

2. Historical Background
3. Material and Methods
3.1. Available Data
3.2. Classification Methods
- ▪ whorl embracement: from evolute to moderately involute;
- ▪ whorl section shape: sub-quadrate or sub-trapezoidal or sub-rectangular;
- ▪ shape of the flanks: more or less rounded;
- ▪ shape of the umbilical wall and ventri-lateral shoulder: rounded or sloping;
- ▪ ventral keel and sulci: variably pronounced and variably carved, respectively;
- ▪ type and shape of the ribs: either simple or paired; paired ribs may merge only at umbilical nodes or even at ventral nodes (forming fibulae), with couplets sometimes formed by a more raised primary rib and an attenuated secondary one. The shape of the ribs is either thick-clavate and overall straight or thin and sinuous, at times also concave backward;
- ▪ number of ribs: less than 10–12 when coarse-clavate, up to more than 20 when thinner and sinuous;
- ▪ umbilical and/or ventro-lateral tubercles and nodes: variably pronounced and overall elongated;
- ▪ suture line: variably simplified, particularly the lobes.
4. Systematic Palaeontology
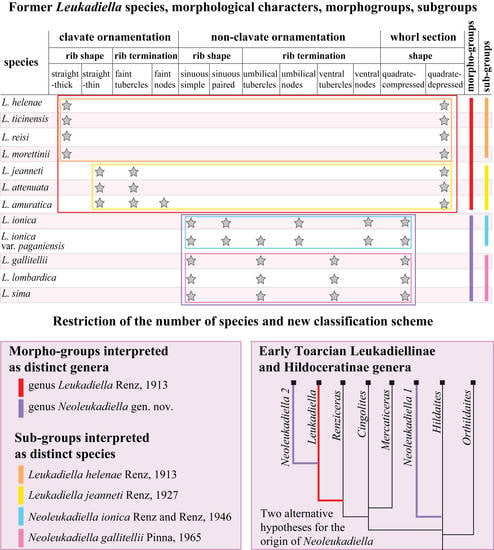
4.1. Morphological Variability of Leukadiella Species and Classification Problems
4.2. Restriction of the Number of Accepted Species
4.3. Revised Classification Scheme
| Subclass Ammonoidea Zittel, 1884 [83] |
| Order Ammonitida Hyatt, 1889 [84] |
| Suborder Ammonitina Hyatt, 1889 [84] |
| Superfamily Hildoceratoidea Hyatt, 1867 [14] |
| Family Hildoceratidae Hyatt, 1867 [14] |
| Subfamily Leukadiellinae Macchioni and Venturi, 2000 [17] |
| Genus Leukadiella Renz, 1913 [15] |
- 1913 Leukadiella helenae; Renz, pl. 14, figures 1–3; text figure 17.
- 1922 Leukadiella helenae var. ticinensis; Renz, pl. 7, figure 1.
- 1923 Leukadiella helenae; Renz, pl. 12, figure 3.
- 1925 Leukadiella reisi; Renz, pl. 5, figure 4a.
- 1927 Leukadiella helenae var. ticinensis; Renz, pl. 13, figure 8a.
- 1966 Leukadiella amuratica; Kottek, pl. 13, figure 7.
- 1966 Leukadiella helenae; Wendt, pl. 13, figures 1a–d, 2a–c, 3a–c, 4a–d.
- 1972 Leukadiella helenae; Levi Setti, text figure III: 2a–d.
- ?1972 Leukadiella sp.; Levi Setti, text figure III: 4a–d.
- 2000 Leukadiella helenae; Macchioni and Venturi, pl. 1, figures 17 and 18; pl. 2, figures 14, 19, 20; pl. 3, figures 14, 20; pl. 4, figures 1, 2.
- 2000 Leukadiella cfr. helenae; Macchioni and Venturi, pl. 1, figures 14–16; pl. 2, figures 11–13; pl. 3, figures 6, 7, 22.
- 2000 Leukadiella morettinii; Macchioni and Venturi, pl. 1, figure 3; pl. 2, figure 10; pl. 3, figures 10, 12–13, 17; text figure 3.
- 2000 Leukadiella aff. morettinii; Macchioni and Venturi, pl. 1, figure 2; pl. 2, figure 15; pl. 3, figure 19.
- ? 2000 Leukadiella aff. jeanneti; Macchioni and Venturi, pl. 1, figure 20; pl. 2, figure 16; pl. 3, figure 15.
- 2008 Leukadiella helenae; Géczy et al., pl. 2, figures 5, 6.
Genus Neoleukadiella gen. nov.
5. Discussion
5.1. Taxonomic Review and Phylogenetic Implications
5.2. Internal Clocks and “Unconventional” Evolutionary Mechanisms
5.3. Pathological Morphotypes and “Coherent Phenotypic Anomaly”
5.4. Environmental Stress, Species Resilience and Biodiversity Recovery
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicosia, U.; Pallini, G. Ammonites and calcareous Nannoplankton of the Toarcian “Rosso Ammonitico” in the exposures of M. La Pelosa (Terni, Central Apennines, Italy). Geol. Romana 1977, 16, 263–283. [Google Scholar]
- Elmi, S. Sédimentation rythmique et organisation séquentielle dans les Ammonitico-Rosso et les faciès associés du Jurassique de la Méditerranée occidentale. Interprétation des grumeaux et des nodules. In Rosso Ammonitico Symposium Proceedings; Farinacci, A., Elmi, S., Eds.; Edizioni Tecnoscienza: Roma, Italy, 1981; pp. 334–343. [Google Scholar]
- Farinacci, A.; Malantrucco, G.; Mariotti, N.; Nicosia, U. Ammonitico Rosso facies in the frame- work of the Martani Mountains paleoenvironmental evolution during Jurassic. In Rosso Ammonitico Symposium Proceedings; Farinacci, A., Elmi, S., Eds.; Edizioni Tecnoscienza: Roma, Italy, 1981; pp. 311–334. [Google Scholar]
- Colacicchi, R.; Passeri, L.; Pialli, G. Nuovi dati sul Giurese Umbro-Marchigiano ed ipotesi per un suo inquadramento regionale. Mem. Soc. Geol. Ital. 1970, 9, 839–874. [Google Scholar]
- Centamore, E.; Chiocchini, M.; Deiana, G.; Micarelli, A.; Pieruccini, V. Contributo alla conoscenza del Giurassico dell’Appennino umbro-marchigiano. Studi Geol. Camerti 1971, 1, 1–89. [Google Scholar]
- Centamore, E.; Cantalamessa, G.; Micarelli, A.; Potetti, M.; Berti, D.; Bigi, S.; Morelli, C.; Ridolfi, M. Stratigrafia e analisi di facies dei depositi del Miocene e del Pliocene inferiore dell’avanfossa marchigiano-abruzzese e delle zone limitrofe. Studi Geol. Camerti 1991, 2, 125–131. [Google Scholar]
- Castellarin, A.; Colacicchi, R.; Praturlon, A. Fasi distensive, trascorrenze e sovrascorrimenti lungo la ‘linea Ancona-Anzio’, dal Lias medio al Pliocene. Geol. Romana 1978, 17, 161–189. [Google Scholar]
- Castellarin, A.; Colacicchi, R.; Praturlon, A.; Cantelli, C. The Jurassic-Lower Pliocene history of the Ancona-Anzio line (Central Italy). Mem. Soc. Geol. It. 1984, 24, 325–336. [Google Scholar]
- Farinacci, A.; Mariotti, N.; Nicosia, U.; Pallini, G.; Schiavinotto, F. Jurassic sediments in the umbro-marchean Apennines: An alternative model. In Rosso Ammonitico Symposium Proceedings; Farinacci, A., Elmi, S., Eds.; Edizioni Tecnoscienza: Roma, Italy, 1981; pp. 335–398. [Google Scholar]
- Cresta, S. Stratigrafia del Triassico-Giurassico. Mem. Descr. Carta Geol. D’italia 1989, 39, 14–22. [Google Scholar]
- Santantonio, M. Facies associations and evolution of pelagic carbonate platform/basin systems: Examples from the Italian Jurassic. Sedimentology 1993, 40, 1039–1067. [Google Scholar] [CrossRef]
- Cipollari, P.; Cosentino, D.; Parotto, M. Modello cinematico-strutturale dell’Italia centrale. Studi Geol. Camerti 1997, 2, 135–143. [Google Scholar]
- Carminati, E.; Corda, L.; Mariotti, G.; Scifoni, A.; Trippetta, F. Mesozoic Syn- and Postrifting Evolution of the Central Apennines, Italy: The Role of Triassic Evaporites. J. Geol. 2013, 121, 327–354. [Google Scholar] [CrossRef]
- Hyatt, A. The fossil Cephalopoda of the Museum of Comparative Zoology. Bull. Mus. Comp. E Zool. 1867, 5, 71–102. [Google Scholar]
- Renz, C. Neue Arten aus dem hellenischen Jura und aus der indischen Dyas. 1. Neue Arten aus dem griechisch epirotischen Oberlias und Unterdogge. Zeitschrift Deutsch. Geol. Gesellsch. 1913, 64, 583–617. [Google Scholar]
- Arkell, W.J. Seven new genera of Jurassic ammonites. Geol. Mag. 1953, 90, 36–40. [Google Scholar] [CrossRef]
- Macchioni, F.; Venturi, F.; Leukadiellinae, N. subfam. of the Lower and Middle Toarcian. Origin and evolution of the genera Renziceras Arkell (1957) and Leukadiella Renz (1913). Boll. Soc. Pal. It. 2000, 39, 319–339. [Google Scholar]
- Thévenin, A. Sur un genre d’Ammonites du Lias de Madagascar. Bull. Soc. Géol. Fr. 1906, 6, 171–173. [Google Scholar]
- Buckman, S.S. Type Ammonites; Part 30; Wheldon & Wesley: London, UK, 1921; Volume 3, pp. 55–64, 16 pls. [Google Scholar]
- Buckman, S.S. Yorkshire Type Ammonites; Parts 9–11; Wesley & Son: London, UK, 1913; Volume 2, pp. V–X, iii–viii, pls 68–90. [Google Scholar]
- Buckman, S.S. Type Ammonites; Part 42; Wheldon & Wesley: London, UK, 1923; Volume 5, 20p. [Google Scholar]
- Kottek, A.V. Die Ammonitenabfolge des griechischen Toarcium. Ann. Géol. Pays Hellén. 1966, 17, 1–157. [Google Scholar]
- Wendt, J. Revision der Ammoniten-Gattung Leukadiella Renz aus dem mediterranen Oberlias. N. J. Gcol. Paläont. Abh. 1966, 125, 136–154. [Google Scholar]
- Guex, J. Les Bouleiceratinae Arkell (Ammonitina, Cephalopoda): Sous-famille mono ou polyphylétique? Eclog. Geol. Helv. 1974, 67, 427–430. [Google Scholar]
- Jakobs, G.K. New occurrences of Leukadiella and Paroniceras (Ammonoidea) from the Toarcian (Lower Jurassic) of the Canadian Cordillera. J. Paleontol. 1995, 69, 89–98. [Google Scholar] [CrossRef]
- Sassaroli, S.; Venturi, F.; Cingolites, N. Gen., a new lower Toarcian Hildoceratinae (Ammonitina) from the Marchean Apennines (Cingoli, Macerata, Italy). Boll. Della Soc. Paleont. Ital. 2010, 49, 97–118. [Google Scholar]
- Courtillot, V. Evolutionary Catastrophes: The Science of Mass Extinction; Cambridge University Press: Cambridge, UK, 1999; p. 173. [Google Scholar]
- Guex, J. Environmental stress and atavism in ammonoid evolution. Eclog. Geol. Helv. 2001, 94, 322–328. [Google Scholar]
- Hesselbo, S.P.; Gröcke, D.R.; Jenkyns, H.C.; Bjerrum, C.J.; Farrimond, P.; Morgans Bell, H.S.; Green, O.R. Massive dissociation of gas hydrate during a Jurassic oceanic anoxic event. Nature 2000, 406, 392–395. [Google Scholar] [CrossRef] [PubMed]
- McArthur, J.M.; Donovan, D.T.; Thirvall, M.F.; Fouke, B.W.; Mattey, D. Strontium isotope profile of the Early Toarcian (Jurassic) oceanic anoxic event, the duration of ammonite biozones, and belemnite palaeotemperatures. Earth Planet. Sci. Lett. 2000, 179, 269–285. [Google Scholar] [CrossRef]
- Mattioli, E.; Pittet, B. Spatial and temporal distribution of calcareous nannofossils along a proximal–distal transect in the Lower Toarcian of the Umbria-Marche basin (central Italy). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 205, 295–316. [Google Scholar] [CrossRef]
- Kemp, D.B.; Coe, A.L.; Cohen, A.S.; Schwark, L. Astronomical pacing of methane release in the Early Jurassic period. Nature 2005, 437, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Beerling, D.J.; Brentnall, S.J. Numerical evaluation of mechanisms driving Early Jurassic changes in global carbon cycling. Geology 2007, 35, 247–250. [Google Scholar] [CrossRef]
- Wignall, P.B.; Newton, R.J.; Little, C.T.S. The timing of paleoenvironmental change and cause-and-effect relationships during the Early Jurassic mass extinction in Europe. Am. J. Sci. 2005, 305, 1014–1032. [Google Scholar] [CrossRef]
- Suan, G.; Pittet, B.; Bour, I.; Mattioli, E.; Duarte, L.V.; Mailliot, S. Duration of the Early Toarcian carbon isotope excursion deduced from spectral analysis: Consequences for its possible causes. Earth Planet. Sci. Lett. 2008, 267, 666–679. [Google Scholar] [CrossRef] [Green Version]
- Sabatino, N.; Neri, R.; Bellanca, A.; Jenkyns, H.C.; Baudin, F.; Parisi, G.; Masetti, D. Carbon-isotope records of the Early Jurassic (Toarcian) oceanic anoxic event from the Valdorbia (Umbria-Marche Apennines) and Monte Mangart (Julian Alps) sections: Palaeoceanographic and stratigraphic implications. Sedimentology 2009, 56, 1307–1328. [Google Scholar] [CrossRef]
- Little, C.T.S.; Benton, M.J. Early Jurassic mass extinction: A global long-term event. Geology 1995, 23, 495–498. [Google Scholar] [CrossRef]
- Jourdan, F.; Féraud, G.; Bertrand, H.; Kampunzu, A.B.; Tshoso, G.; Watkeys, M.K.; Le Gall, B. Karoo large igneous province: Brevity, origin, and relation to mass extinction questioned by new 40Ar/39Ar age data. Geology 2005, 33, 745–748. [Google Scholar] [CrossRef] [Green Version]
- Jourdan, F.; Féraud, G.; Bertrand, H.; Watkeys, M.K.; Renne, P.R. Distinct brief major events in the Karoo large igneous province clarified by new 40Ar/39Ar ages on the Lesotho basalts. Lithos 2007, 98, 195–209. [Google Scholar] [CrossRef]
- Hallam, A.; Wignall, P.B. Mass extinctions and sea-level changes. Earth-Sci. Rev. 1999, 48, 217–250. [Google Scholar] [CrossRef]
- Sandoval, J.; O’Dogherthy, L.; Vera, J.A.; Guex, J. Sea level changes and ammonite faunal turnover during the Lias/Dogger transition in the western Tethys. Bull. Soc. Géol. Fr. 2002, 173, 57–66. [Google Scholar] [CrossRef]
- Macchioni, F.; Cecca, F. Biodiversity and biogeography of middle-late liassic ammonoids: Implications for the Early Toarcian mass extinction. Geobios 2002, 35, 150–164. [Google Scholar] [CrossRef]
- Cecca, F.; Macchioni, F. The two Early Toarcian (Early Jurassic) extinction events in ammonoids. Lethaia 2004, 37, 35–56. [Google Scholar] [CrossRef]
- Guex, J. Reinitialization of evolutionary clocks during sublethal environmental stress in some invertebrates. Earth Planet. Sci. Lett. 2006, 242, 240–253. [Google Scholar] [CrossRef]
- Dera, G.; Neige, P.; Dommergues, J.L.; Fara, E.; Laffont, R.; Pellenard, P. High-resolution dynamics of Early Jurassic marine extinctions: The case of Pliensbachian-Toarcian ammonites (Cephalopoda). J. Geol. Soc. 2010, 167, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, A.A.; Hercus, M.J. Environmental Stress as an Evolutionary Force. BioScience 2000, 50, 217–226. [Google Scholar] [CrossRef]
- Gilbert, S.F.; Epel, D. Ecological Developmental Biology: Integrating Epigenetics, Medicine, and Evolution; Sinauer Associates Inc.: Sunderland, MA, USA, 2008; p. 459. [Google Scholar]
- Nicoglou, A.; Merlin, F. Epigenetics: A way to bridge the gap between biological fields. Stud. Hist. Philos. Biol. Biomed. Sci. 2017, 66, 73–78. [Google Scholar] [CrossRef]
- Renz, C. Einige Tessiner Oberlias-Ammoniten. Eclog. Geol. Helv. 1922, 17, 137–166. [Google Scholar]
- Renz, C.; Renz, O. Einige seltene Ammoniten aus dem griechischen Mesozoikum. Eclog. Geol. Helv. 1946, 39, 169–176. [Google Scholar]
- Renz, C. Paroniceraten, Frechiellen und Leukadiellen der österreichischen und bayerischen Alpen, nebst schwäbischen und französischen Vcrgleichsstückcn. Verh. Nat. Ges. 1925, 36, 200–219. [Google Scholar]
- Renz, C. Frechiellen, Leukadiellen und Paroniceraten im westgriechischen Oberlias mit tessinischen Vergleichsstückcn. Eclog. Geol. Helv. 1927, 20, 422–444. [Google Scholar]
- Pinna, G. Nuove specie di Ammoniti del genere Leukadiella del Toarciano inferiore delle Foci del Burano (Umbria) e delle Alpe Turati (Lombardia). Boll. Soc. Geol. It. 1965, 84, 268–277. [Google Scholar]
- Lehmann, J.; Von Bargen, D.; Engelke, G.; Claßen, J. Morphological variability in response to paleonvironmental change—A case study on Cretaceous. Lethaia 2016, 49, 73–86. [Google Scholar] [CrossRef]
- Bilotta, M.; Venturi, F.; Sassaroli, S. Ammonite faunas, OAE and the Pliensbachian Toarcian boundary (Early Jurassic) in the Apennines. Lethaia 2010, 43, 357–380. [Google Scholar] [CrossRef]
- Braga, J.C.; Jiménez, A.P.; Rivas, P. Ammonites (Bouleiceratinés) à signification paléogèographique du Toarcien de la Zone Subbétique (Sud de l’Espagne). Compt. Rend. Acad. Sci. Paris 1985, 8, 533–555. [Google Scholar]
- Hillebrandt, A. von. Kontinentalverschiebung und die paläzoogeographischen Beziehungen des südamerikanischen Lias. Geol. Rundsch. 1981, 70, 570–582. [Google Scholar] [CrossRef]
- Prinz, G. Die Fauna der alteren Jurabildungen im nordöstlichen Bakony. Mitteil. Aus Dem Jahrb. Der Königlich Ung. Geol. Anst. 1904, 15, 142. [Google Scholar]
- Bonarelli, G. Osservazioni sul Toarciano e l’Aleniano dell’Appennino Centrale. Boll. Soc. Geol. It. 1893, 12, 195–254. [Google Scholar]
- Arkell, W.J. Jurassic ammonites from Jebel Tuwaiq, central Arabia. Philos. Trans. Roy. Soc. Lond. 1952, 236, 241–313. [Google Scholar]
- Goy, A.; Martínez, G. Paroniceratinae (Ammonoidea, Hildoceratidae) del Toarciense en las Cordilleras Ibérica y Cantábrica (España). Geobios 2009, 42, 603–622. [Google Scholar] [CrossRef] [Green Version]
- Kovács, Z. Paroniceratidae (Ammonitina) of the Toarcian from the Gerecse Mts (NE Transdanubian Range, Hungary). Földtani Közlöny 2010, 140, 119–134. [Google Scholar]
- Martínez, G.; Garcia-Joral, F. Bouleiceras (Hildoceratidae, Ammonitina) from the lower Toarcian (Jurassic) of the Iberian Range (Spain): Taxonomy, stratigraphic distribution and insights on its dispersal. Geobios 2020, 62, 31–44. [Google Scholar] [CrossRef]
- Goy, A.; Comas-Rengifo, M.J. Nejdia (Hildoceratidae, Ammonoidea) in the Toarcian (Lower Jurassic) of the Iberian Peninsula. Systematics, phylogenetic affinities and palaeobiogeography. Hist. Biol. 2021, 33, 3173–3190. [Google Scholar] [CrossRef]
- Howarth, M.K. Treatise Online, Part L, Revised, Volume 3B, Chapter 4: Psiloceratoidea, Eodoceratoidea, Hildoceratoidea; Geological Society of America and University of Kansas: Lawrence, KS, USA, 2013; Volume 57, p. 139. [Google Scholar]
- Rulleau, L.; Bécaud, M.; Neige, P. Les ammonites traditionnellement regroupées dans la sous-famille des Bouleiceratinae (Hildoceratidae, Toarcien): Aspects phylogénétiques, biogéographiques et systématiques. Geobios 2003, 36, 317–348. [Google Scholar] [CrossRef]
- Goy, A.; Martínez, G. Distribución bioestratigráfica de los Bouleiceratinae en las Cuencas Ibérica y Vasco-Cantábrica. Real Soc. Esp. Hist. Nat. 1996, 125, 306–310. [Google Scholar]
- Haug, E. Note sur quelques éspèces d’ammonites nouvelles ou peu connues du Lias supérieur. Bull. Soc. Géol. Fr. 1884, 12, 346–356. [Google Scholar]
- Gabilly, J. Le Toarcien à Thouars et dans le centre-ouest de la France. Strat. Français 1976, 3, 217. [Google Scholar]
- Guex, J. Aperçu biostratigraphique sur le Toarcien inférieur du Moyen-Atlas marocain et discussion sur la zonation de ce sous-étage dans les séries méditerranéennes. Eclog. Geol. Helv. 1973, 66, 493–523. [Google Scholar]
- Renz, C. Vergleiche zwischen dem südschweizerischen, apenninischen und westgriechischen Jura. Verh. naturforsch. Ges. 1923, 34, 264–296. [Google Scholar]
- Gallitelli, M.F. Ritrovamento di un’ammonite del gen. Bouleiceras Thévenin nel Toarciano dell’Appennino centrale. Boll. Soc. Paleont. It. 1963, 2, 107–110. [Google Scholar]
- Gallitelli-Wendt, M.F. Ammoniti e Stratigrafia del Toarciano umbro-marchigiano (Appennino centrale). Boll. Soc. Paleont. It. 1969, 8, 11–62. [Google Scholar]
- Deleau, P. Le Djebel Nador. Études stratigraphique et paléontologique. En appendice: Note géologique sur les Monts de Chellala et aperçu sur l’hydrologie du Djebel Nador et des Monts de Chellala. Bull. Serv. Carte géol. Algérie 1948, 2, 126. [Google Scholar]
- Schindewolf, O.H. Studien zur Stammesgeschichte der Ammoniten. Akad. Der Wissen. Und Der Liter. 1963, 6, 287–432. [Google Scholar]
- Levi Setti, F. Nuovi rinvenimenti di ammoniti toarciane del genere Leukadiella nell’Appennino Centrale e nelle Alpi Lombarde. Soc. It. Sc. Nat. Mus. Civ. Stor. Nat. Acquar. Civ. Milano 1972, 63, 37–46. [Google Scholar]
- Howarth, M.K. The ammonite family Hildoceratidae in the Lower Jurassic of Britain (Part 1-2). Paleontogr. Soc. Monogr. 1992, 145–146, 1–200. [Google Scholar]
- Pettinelli, R.; Nocchi, M.; Parisi, G. Late Pliensbachian-Toarcian biostratigraphy and environmental interpretations in the Ionian Basin (Lefkas Island, Western Greece) as compared to the Umbria-Marchean Basin (Central Italy). Bollett. Serv. Geol. 1997, 114, 97–158. [Google Scholar]
- Géczy, B.; Szente, I. Middle Toarcian Ammonitina from the Gerecse Mts, Hungary. Acta Geol. Hung. 2007, 49, 223–252. [Google Scholar] [CrossRef]
- Géczy, B.; Kovács, Z.; Szente, I. Remarks on the Toarcian–Aalenian fossil assemblage of the Kis-Teke Hill, Gerecse Mts (Hungary). Hantkeniana 2008, 6, 33–55. [Google Scholar]
- Xu, W.; MacNiocaill, C.; Ruhl, M.; Jenkyns, H.C.; Riding, J.B.; Hesselbo, S.P. Magnetostratigraphy of the Toarcian Stage (Lower Jurassic) of the Llanbedr (Mochras Farm) Borehole, Wales: Basis for a global standard and implications for volcanic forcing of palaeoenvironmental change. J. Geol. Soc. 2018, 175, 594–604. [Google Scholar] [CrossRef]
- Meister, E. Zur Kenntnis der Ammonitenfauna des Portugiesischen Lias. Zeitsch. der Deutsch. Geol, Gesell. 1913, 65, 518–586. [Google Scholar]
- Zittel, K.A. von. Handbuch der Paläontologie; München und Leipzig: Munich, Germany, 1884; Volume 2, p. 893. [Google Scholar]
- Hyatt, A. Genesis of the Arietitidae. Smithson. Contrib. Knowl. 1889, 673, 238. [Google Scholar]
- Merla, G. Ammoniti giuresi dell’Appennino Centrale. 1, Hildoceratidae. Palaeontogr. Ital. 1933, 33, 1–54. [Google Scholar]
- Gabilly, J. Paléobiogéographie et taxonomie des Hildocerataceae (Ammonitina) du Toarcien. Compt. Rend. Séance l’Acad. Sci. 1974, 279, 1245–1248. [Google Scholar]
- Buckman, S.S. A Monograph of the Inferior Oolite Ammonites of the British Islands; Part 10; Palaeontographical Society: London, UK, 1898; pp. i–xxxii, pls 1–4. [Google Scholar]
- Monnet, C.; Bucher, H.; Brayard, A.; Jenks, J.F. Globacrochordiceras gen. nov. (Acrochordiceratidae, late Early Triassic) and its significance for stress-induced evolutionary jumps in ammonoid lineages (cephalopods). Foss. Rec. 2013, 16, 197–215. [Google Scholar] [CrossRef]
- Kummel, B. Environmental significance of dwarfed cephalopods. J. Sed. Res. 1948, 18, 61–64. [Google Scholar] [CrossRef]
- Mignot, Y.; Elmi, S.; Dommergues, J.-L. Croissance et miniaturisation de quelques Hildoceras (Cephalopoda) en liaison avec des environnements contraignants de la Téthys toarcienne. Geobios 1993, 15, 305–312. [Google Scholar] [CrossRef]
- Urlichs, M. Stunting in some invertebrates from the Cassian Formation (Late Triassic, Carnian) of the Dolomites (Italy). N. J. Gcol. Paläont. Abh. 2012, 265, 1–25. [Google Scholar] [CrossRef]
- Hoffmann, R.; Keupp, H. Ammonoid Paleopathology. In Ammonoid Paleobiology: From Anatomy to Ecology; Klug, C., Korn, D., De Baets, K., Kruta, I., Mapes, R.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 43, pp. 887–996. [Google Scholar]
- Jattiot, R.; Fara, E.; Brayard, A.; Urdy, S.; Goudemand, G. Learning from beautiful monsters: Phylogenetic and morphogenetic implications of left-right asymmetry in ammonoid shells. BMC Evol. Biol. 2019, 19, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, S.F. The morphogenesis of evolutionary developmental biology. Int. J. Dev. Biol. 2003, 47, 467–477. [Google Scholar]
- Badyaev, A.V. Role of stress in evolution:from individual adaptabilityto evolutionary adaptation. In Variation; Hallgrimson, B., Hall, B.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 277–302. [Google Scholar]
- Buckman, S.S. Type Ammonites; Part 69; Wheldon & Wesley: London, UK, 1928; Volume 7, pp. 11–14, 18 pls. [Google Scholar]
- Bruguière, J.G. Histoire Naturelle des vers. Enciclopeédie Méthod. 1789, 6, 1–344. [Google Scholar]
- Bellini, R. Les Ammonites du Calcaire Rouge Ammonitique (Toarcien) de l’Ombrie. J. Conchol. 1900, 48, 122–164. [Google Scholar]
- Neige, P.; Dera, G.; Dommergues, J.-L. Adaptive radiation in the fossil record: A case study among Jurassic Ammonoids. Palaeontology 2013, 56, 1247–1261. [Google Scholar] [CrossRef]
- Donovan, D.T. The Ammonite zones of the Toarcian (Ammonitico Rosso facies) of Southern Switzerland and Italy. Eclogae Geol. Helv. 1958, 51, 33–60. [Google Scholar]
- Ridente, D. Heterochrony and evolution in some Toarcian ammonites. Speculations and insights. J. Mediterr. Earth Sci. 2016, 8, 25–37. [Google Scholar]
- Ridente, D. The “grooved Hildoceras ammonite fauna from the Lower Jurassic (Toarcian) of the Apennines. Overview and proposal for a new species. Hist. Biol. 2022. [Google Scholar] [CrossRef]











| Author(s) | Number of Specimens | Number of Species | Findings from Italian Localities |
|---|---|---|---|
| Renz [13] | 1 | 1 L. helenae n. sp. | |
| Renz [49] | 1 | 1 L. helenae n. v. ticinensis | |
| Renz [71] | 1 | 1 L. helenae | |
| Renz [51] | 2 | 1 L. reisi n. sp.; 1 L. helenae var. | |
| Renz [52] | 2 | 1 L. helenae v. ticinensis; 1 L. jeanneti n. sp. | |
| Renz and Renz [50] | 5 | 1 L. amuratica n. sp.; 3 L. ionica n. sp.; 1 L. ionica n.v. paganiensis | |
| Deleau [74] (fide Wendt [23]) | 1 | 1 L. ionica | |
| Schindewolf [75] (fide Wendt [23] (p.142,145)) | 2 | 1 L. ionica; 1 L. sp. | |
| Pinna [53] | 2 | 1 L. gallitellii n. sp.; 1 L. lombardica | 1 L. gallitellii (Central Apennines) 1 L. lombardica (Southern Alps) |
| Kottek [22] | 5 | 1 L. amuratica; 1 L. ionica; 1 L. sima n. sp.; 1 L. paganiensis; 1 L. ionica subsp. | |
| Wendt [23] | 6 | 2 L. helenae; 1 L. ionica; 2 L. attenuata n. sp.; 1 L. sp.; 3 L. ionica (not figured, not counted herein) | 1 L. ionica (Central Apennines) 2 L. helenae (Sicily) 1 L. attenuata n. sp. (Sicily) |
| Gallitelli-Wendt [73] | 1 | 1 L. ionica (same specimen figured by Wendt [23]; counted therein) | |
| Levi Setti [76] | 4 | 1 L. helenae, 1 L. ionica, 1 L. lombardica, 1 L. sp. | 1 L. ionica (Central Apennines) 1 L. helenae (Central Apennines) 1 L. sp. (Central Apennines) 1 L. lombardica (Southern Alps) |
| Hillebrandt [57] (fide Jakobs [25]) | 1 (doubtful) | 1? | |
| Howarth [77] | 1 | 1 L. aff. ionica (doubtful) | |
| Jakobs [25] | 14 | 8 L. ionica; 2 L. aff. ionica; 2 L. amuratica; 1 L. aff. helenae; 1 L. sp. | |
| Pettinelli et al. [78] | 1 | 1 L. ionica | |
| Macchioni and Venturi [17] | 17 | 3 L. helenae; 3 L. cfr. helenae; 2 L. morettinii n. sp.; 1 L. aff. morettinii; 3 L. ionica; 3 L. gallitellii; 1 L. aff. jeanneti; 1 L. n. sp. | All from the Central Apennines |
| Géczy and Szente [79] | 1 | 1 L. jeanneti | |
| Géczy et. al. [80] | 1 | 1 L. helenae | |
| Ridente, this work | 2 | 2 L. helenae | Both from the Central Apennines |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ridente, D. On the Rarity and Peculiarity of the Early Toarcian (Lower Jurassic) Leukadiellinae Ammonites—Systematic Review and Insights on the Interplay of Environmental Stress, Evolution and Biodiversity. Geosciences 2022, 12, 411. https://doi.org/10.3390/geosciences12110411
Ridente D. On the Rarity and Peculiarity of the Early Toarcian (Lower Jurassic) Leukadiellinae Ammonites—Systematic Review and Insights on the Interplay of Environmental Stress, Evolution and Biodiversity. Geosciences. 2022; 12(11):411. https://doi.org/10.3390/geosciences12110411
Chicago/Turabian StyleRidente, Domenico. 2022. "On the Rarity and Peculiarity of the Early Toarcian (Lower Jurassic) Leukadiellinae Ammonites—Systematic Review and Insights on the Interplay of Environmental Stress, Evolution and Biodiversity" Geosciences 12, no. 11: 411. https://doi.org/10.3390/geosciences12110411
APA StyleRidente, D. (2022). On the Rarity and Peculiarity of the Early Toarcian (Lower Jurassic) Leukadiellinae Ammonites—Systematic Review and Insights on the Interplay of Environmental Stress, Evolution and Biodiversity. Geosciences, 12(11), 411. https://doi.org/10.3390/geosciences12110411