The Role of Climate: 71 ka of Atmospheric Mercury Deposition in the Southern Hemisphere Recorded by Rano Aroi Mire, Easter Island (Chile)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Regional Setting
2.2. Sampling
2.3. Age Model
2.4. Geochemical Analysis
3. Results
3.1. Organic Fraction: Total Carbon, Total Nitrogen, C/N and δ13C
3.2. Mercury
4. Discussion
4.1. Mercury Concentrations in Rano Aroi Records
4.2. Environmental Conditions in Rano Aroi and Related Proxies
4.3. Processes Controlling Hg Content
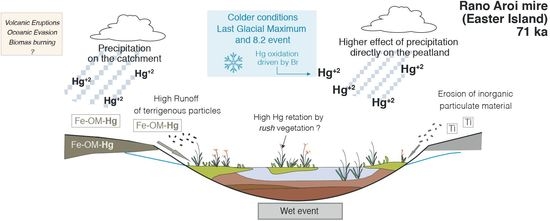
4.3.1. Climate
Temperature
Effective Moisture
Dry vs. Wet Deposition in Rano Aroi
4.3.2. Effect of Catchment Erosion: Geologic Materials and Catchment Soils
4.3.3. Other Processes: Mire Vegetation, Peat Decomposition, Aeolian Dust, Oceanic Evasion, and Volcanic Activity
Mire Vegetation
Peat Decomposition
Aeolian Dust
Oceanic Evasion
Volcanic Activity
Fire Regime
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schroeder, W.H.; Munthe, J. Atmospheric mercury—An overview. Atmos. Environ. 1998, 32, 809–822. [Google Scholar] [CrossRef]
- Hylander, L.D.; Meili, M. The Rise and Fall of Mercury: Converting a Resource to Refuse After 500 Years of Mining and Pollution. Crit. Rev. Environ. Sci. Technol. 2005, 35, 1–36. [Google Scholar] [CrossRef]
- Horowitz, H.M.; Jacob, D.J.; Amos, H.M.; Streets, D.G.; Sunderland, E.M. Historical mercury releases from commercial products: Global environmental implications. Environ. Sci. Technol. 2014, 48, 10242–10250. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Saiz-Lopez, A.; Mahajan, A.S.; Gómez Martín, J.C.; Armstrong, D.; Lemes, M.; Hay, T.; Prados-Roman, C. Enhanced production of oxidised mercury over the tropical Pacific Ocean: A key missing oxidation pathway. Atmos. Chem. Phys. 2014, 14, 1323–1335. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, W.F.; Mason, R.P. The global mercury cycle: Oceanic and anthropogenic aspects. In Global and Regional Mercury Cycles: Sources, Fluxes and Mass Balances; Springer: Berlin/Heidelberg, Germany, 1996; pp. 85–108. [Google Scholar]
- Amos, H.M.; Sonke, J.E.; Obrist, D.; Robins, N.; Hagan, N.; Horowitz, H.M.; Mason, R.P.; Witt, M.; Hedgecock, I.M.; Corbitt, E.S.; et al. Observational and modeling constraints on global anthropogenic enrichment of mercury. Environ. Sci. Technol. 2015, 49, 4036–4047. [Google Scholar] [CrossRef] [PubMed]
- Biester, H.; Bindler, R.; Martinez-Cortizas, A.; Engstrom, D.R. Modeling the Past Atmospheric Deposition of Mercury Using Natural Archives. Environ. Sci. Technol. 2007, 41, 4851–4860. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, D.R.; Fitzgerald, W.F.; Cooke, C.A.; Lamborg, C.H.; Drevnick, P.E.; Swain, E.B.; Balogh, S.J.; Balcom, P.H. Atmospheric Hg emissions from preindustrial gold and silver extraction in the Americas: A reevaluation from lake-sediment archives. Environ. Sci. Technol. 2014, 48, 6533–6543. [Google Scholar] [CrossRef] [PubMed]
- Cloy, J.M.; Farmer, J.G.; Graham, M.C.; MacKenzie, A.B.; Cook, G.T. Historical records of atmospheric Pb deposition in four Scottish ombrotrophic peat bogs: An isotopic comparison with other records from western Europe and Greenland. Glob. Biogeochem. Cycles 2008, 22. [Google Scholar] [CrossRef] [Green Version]
- Zaferani, S.; Pérez-Rodríguez, M.; Biester, H. Diatom ooze—A large marine mercury sink. Science 2018, eaat2735. [Google Scholar] [CrossRef] [PubMed]
- Lamborg, C.H.; Fitzgerald, W.F.; Damman, A.W.H.; Benoit, J.M.; Balcom, P.H.; Engstrom, D.R. Modern and historic atmospheric mercury fluxes in both hemispheres: Global and regional mercury cycling implications. Glob. Biogeochem. Cycles 2002, 16, 1104. [Google Scholar] [CrossRef]
- Biester, H.; Martinez-Cortizas, A.; Birkenstock, S.; Kilian, R. Effect of Peat Decomposition and Mass Loss on Historic Mercury Records in Peat Bogs from Patagonia. Environ. Sci. Technol. 2003, 37, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Biester, H.; Keppler, F.; Putschew, A.; Martinez-Cortizas, A.; Petri, M. Halogen Retention, Organohalogens, and the Role of Organic Matter Decomposition on Halogen Enrichment in Two Chilean Peat Bogs. Environ. Sci. Technol. 2004, 38, 1984–1991. [Google Scholar] [CrossRef] [PubMed]
- Martínez Cortizas, A.; Pontevedra-Pombal, X.; García-Rodeja, E.; Nóvoa-Muñoz, J.C.; Shotyk, W. Mercury in a Spanish Peat Bog: Archive of Climate Change and Atmospheric Metal Deposition. Science 1999, 284, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Jitaru, P.; Gabrielli, P.; Marteel, A.; Plane, J.M.C.; Planchon, F.A.M.; Gauchard, P.-A.; Ferrari, C.P.; Boutron, C.F.; Adams, F.C.; Hong, S.; et al. Atmospheric depletion of mercury over Antarctica during glacial periods. Nat. Geosci. 2009, 2, 505–508. [Google Scholar] [CrossRef]
- Outridge, P.M.; Sanei, H.; Stern, G.A.; Hamilton, P.B.; Goodarzi, F. Evidence for control of mercury accumulation rates in Canadian High Arctic Lake sediments by variations of aquatic primary productivity. Environ. Sci. Technol. 2007, 41, 5259–5265. [Google Scholar] [CrossRef] [PubMed]
- Kirk, J.L.; Muir, D.C.M.; Antoniades, D.; Douglas, M.S.V.; Evans, M.S.; Jackson, T.A.; Kling, H.; Amoureux, S.; Lim, D.S.S.; Pienitz, R.; et al. Climate change and mercury accumulation in canadian high and subarctic lakes. Environ. Sci. Technol. 2011, 45, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Biester, H.; Pérez-Rodríguez, M.; Gilfedder, B.-S.; Martínez Cortizas, A.; Hermanns, Y.-M. Solar irradiance and primary productivity controlled mercury accumulation in sediments of a remote lake in the Southern Hemisphere during the past 4000 years: Primary productivity and mercury accumulation. Limnol. Oceanogr. 2018, 63, 540–549. [Google Scholar] [CrossRef]
- Rydberg, J.; Klaminder, J.; Rosén, P.; Bindler, R. Climate driven release of carbon and mercury from permafrost mires increases mercury loading to sub-arctic lakes. Sci. Total Environ. 2010, 408, 4778–4783. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Guevara, S.; Meili, M.; Rizzo, A.; Daga, R.; Arribére, M. Sediment records of highly variable mercury inputs to mountain lakes in Patagonia during the past millennium. Atmos. Chem. Phys. 2010, 10, 3443–3453. [Google Scholar] [CrossRef] [Green Version]
- Daga, R.; Ribeiro Guevara, S.; Pavlin, M.; Rizzo, A.; Lojen, S.; Vreča, P.; Horvat, M.; Arribére, M. Historical records of mercury in southern latitudes over 1600 years: Lake Futalaufquen, Northern Patagonia. Sci. Total Environ. 2016, 553, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Roos-Barraclough, F.; Martinez-Cortizas, A.; García-Rodeja, E.; Shotyk, W.; García-Rodeja, E.; Shotyk, W. A 14,500 year record of the accumulation of atmospheric mercury in peat: Volcanic signals, anthropogenic influences and a correlation to bromine accumulation. Earth Planet. Sci. Lett. 2002, 202, 435–451. [Google Scholar] [CrossRef]
- Schuster, P.F.; Krabbenhoft, D.P.; Naftz, D.L.; Cecil, L.D.; Olson, M.L.; Dewild, J.F.; Susong, D.D.; Green, J.R.; Abbott, M.L. Atmospheric Mercury Deposition during the Last 270 Years: A Glacial Ice Core Record of Natural and Anthropogenic Sources. Environ. Sci. Technol. 2002, 36, 2303–2310. [Google Scholar] [CrossRef] [PubMed]
- Corella, J.P.; Wang, F.; Cuevas, C.A. 700 years reconstruction of mercury and lead atmospheric deposition in the Pyrenees (NE Spain). Atmos. Environ. 2017, 155, 97–107. [Google Scholar] [CrossRef]
- Hermanns, Y.; Biester, H. Anthropogenic mercury signals in lake sediments from southernmost Patagonia, Chile. Sci. Total Environ. 2013, 445–446, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, Y.-M.; Cortizas, A.M.; Arz, H.; Stein, R.; Biester, H. Untangling the influence of in-lake productivity and terrestrial organic matter flux on 4250 years of mercury accumulation in Lake Hambre, Southern Chile. J. Paleolimnol. 2012, 49, 563–573. [Google Scholar] [CrossRef]
- Franzen, C.; Kilian, R.; Biester, H. Natural mercury enrichment in a minerogenic fen--evaluation of sources and processes. J. Environ. Monit. 2004, 6, 466–472. [Google Scholar] [CrossRef] [PubMed]
- De Lacerda, L.D.; Turcq, B.; Sifeddine, A.; Cordeiro, R.C. Mercury accumulation rates in Caço Lake, NE Brazil during the past 20,000 years. J. S. Am. Earth Sci. 2017, 77, 42–50. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, M.; Horák-Terra, I.; Rodríguez-Lado, L.; Aboal, J.R.; Martínez Cortizas, A. Long-Term (~57 ka) controls on mercury accumulation in the souther hemisphere reconstructed using a peat record from pinheiro mire (Minas Gerais, Brazil). Environ. Sci. Technol. 2015, 49, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, M.; Horák-Terra, I.; Rodríguez-Lado, L.; Martínez Cortizas, A. Modelling mercury accumulation in minerogenic peat combining FTIR-ATR spectroscopy and partial least squares (PLS). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 168, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Margalef, O.; Cañellas-Boltà, N.; Pla-rabes, S.; Giralt, S.; Pueyo, J.J.; Joosten, H.; Rull, V.; Buchaca, T.; Hernández, A.; Valero-Garcés, B.L.; et al. 70,000 year multiproxy record of climatic and environmental change from Rano Aroi peatland (Easter Island). Glob. Planet. Chang. 2013, 108, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Margalef, O.; Martínez Cortizas, A.; Kylander, M.; Pla-Rabes, S.; Cañellas-Boltà, N.; Pueyo, J.J.; Sáez, A.; Valero-Garcés, B.L.; Giralt, S. Environmental processes in Rano Aroi (Easter Island) peat geochemistry forced by climate variability during the last 70 kyr. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 414, 438–450. [Google Scholar] [CrossRef] [Green Version]
- Baker, P.E.; Buckley, F.; Holland, J.G. Petrology and geochemistry of Easter Island. Contrib. Mineral. Petrol. 1974, 44, 85–100. [Google Scholar] [CrossRef]
- González-Ferrán, O.; Mazzuoli, R.; Lahsen, A. Geología del Complejo Volcánico Isla de Pascua, Rapa Nui, Chile: V Region-Valparaiso; Centro de Estudio Volcanológicos de Santiago: Santiago, Chile, 2004. [Google Scholar]
- Sáez, A.; Valero-Garcés, B.L.; Giralt, S.; Moreno, A.; Bao, R.; Pueyo, J.J.; Hernández, A.; Casas, D. Glacial to Holocene climate changes in the SE Pacific. The Raraku Lake sedimentary record (Easter Island, 27 S). Q. Sci. Rev. 2009, 28, 2743–2759. [Google Scholar] [CrossRef]
- Junk, C.; Claussen, M. Simulated climate variability in the region of Rapa Nui during the last millennium. Clim. Past 2011, 7, 579–586. [Google Scholar] [CrossRef] [Green Version]
- Zizka, G. Flowering plants of Easter Island. In Palmarum Hortus Francofurtensis; Palmengarten: Frankfurt am Main, Germany, 1991. [Google Scholar]
- Flenley, J.R.; King, S.M. Late Quaternary pollen records from Easter Island. Nature 1984, 307, 47–50. [Google Scholar] [CrossRef]
- Flenley, J.R.; King, A.S.M.; Jackson, J.; Chew, C.; Teller, J.T.; Prentice, M.E. The Late Quaternary vegetational and climatic history of Easter Island. J. Quat. Sci. 1991, 6, 85–115. [Google Scholar] [CrossRef]
- Reimer, P.J.; Baillie, M.G.L.; Bard, E.; Bayliss, A.; Beck, J.W.; Bertrand, C.J.H.; Blackwell, P.G.; Buck, C.E.; Burr, G.S.; Cutler, K.B. IntCal04 terrestrial radiocarbon age calibration, 0–26 cal kyr BP. Radiocarbon 2004, 46, 1029–1058. [Google Scholar] [CrossRef]
- Danzeglocke, U.; Jöris, O.; Weninger, B. CalPal-2007. Available online: http://www.calpal-online.de (accessed on 3 May 2009).
- Steinnes, E.; Rühling, Å.; Lippo, H.; Mäkinen, A. Reference materials for large-scale metal deposition surveys. Accredit. Qual. Assur. 1997, 2, 243–249. [Google Scholar] [CrossRef]
- Martínez Cortizas, A.; Peiteado Varela, E.; Bindler, R.; Biester, H.; Cheburkin, A. Reconstructing historical Pb and Hg pollution in NW Spain using multiple cores from the Chao de Lamoso bog (Xistral Mountains). Geochim. Cosmochim. Acta 2012, 82, 68–78. [Google Scholar] [CrossRef]
- Bindler, R.; Klarqvist, M.; Klaminder, J.; Förster, J. Does within-bog spatial variability of mercury and lead constrain reconstructions of absolute deposition rates from single peat records? The example of Store Mosse, Sweden. Glob. Biogeochem. Cycles 2004, 18, 1–12. [Google Scholar] [CrossRef]
- Zuna, M.; Ettler, V.Š.O.; Mihaljevi, M.; Šebek, O.; Mihaljevič, M. Mercury accumulation in peatbogs at Czech sites with contrasting pollution histories. Sci. Total Environ. 2012, 424, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Farmer, J.G.; Anderson, P.; Cloy, J.M.; Graham, M.C.; MacKenzie, A.B.; Cook, G.T. Historical accumulation rates of mercury in four Scottish ombrotrophic peat bogs over the past 2000 years. Sci. Total Environ. 2009, 407, 5578–5588. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Rose, N.; Boyle, J.; Battarbee, R. Storage and distribution of trace metals and spheroidal carbonaceous particles (SCPs) from atmospheric deposition in the catchment peats of Lochnagar, Scotland. Environ. Pollut. 2001, 115, 231–238. [Google Scholar] [CrossRef]
- Tang, S.; Huang, Z.; Liu, J.; Yang, Z.; Lin, Q. Atmospheric mercury deposition recorded in an ombrotrophic peat core from Xiaoxing’an Mountain, Northeast China. Environ. Res. 2012, 118, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Outridge, P.M.; Rausch, N.; Percival, J.B.; Shotyk, W.; McNeely, R. Comparison of mercury and zinc profiles in peat and lake sediment archives with historical changes in emissions from the Flin Flon metal smelter, Manitoba, Canada. Sci. Total Environ. 2011, 409, 548–563. [Google Scholar] [CrossRef] [PubMed]
- Allan, M.; Le Roux, G.; Sonke, J.E.; Piotrowska, N.; Streel, M.; Fagel, N. Reconstructing historical atmospheric mercury deposition in Western Europe using: Misten peat bog cores, Belgium. Sci. Total Environ. 2013, 442, 290–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rull, V.; Cañellas-Boltà, N.; Margalef, O.; Sáez, A.; Pla-Rabes, S.; Giralt, S. Late Holocene vegetation dynamics and deforestation in Rano Aroi: Implications for Easter Island’s ecological and cultural history. Q. Sci. Rev. 2015, 126, 219–226. [Google Scholar] [CrossRef]
- Saiz-Lopez, A.; von Glasow, R. Reactive halogen chemistry in the troposphere. Chem. Soc. Rev. 2012, 41, 6448–6472. [Google Scholar] [CrossRef] [PubMed]
- Goodsite, M.E.; Plane, J.M.C.; Skov, H. A Theoretical Study of the Oxidation of Hg0 to HgBr2 in the Troposphere. Environ. Sci. Technol. 2004, 38, 1772–1776. [Google Scholar] [CrossRef] [PubMed]
- Alley, R.B.; Mayewski, P.A.; Sowers, T.; Stuiver, M.; Taylor, K.C.; Clark, P.A. Holocene climate variability: A prominent widespread event 8200 years ago. Geology 1997, 25, 483–486. [Google Scholar] [CrossRef]
- Enrico, M.; Le Roux, G.; Marusczak, N.; Heimbürger, L.-E.; Claustres, A.; Fu, X.; Sun, R.; Sonke, J.E. Atmospheric Mercury Transfer to Peat Bogs Dominated by Gaseous Elemental Mercury Dry Deposition. Environ. Sci. Technol. 2016, acs.est.5b06058. [Google Scholar] [CrossRef] [PubMed]
- Selin, N.E.; Jacob, D.J.; Yantosca, R.M.; Strode, S.; Jaeglé, L.; Sunderland, E.M. Global 3-D land-ocean-atmosphere model for mercury: Present-day versus preindustrial cycles and anthropogenic enrichment factors for deposition. Glob. Biogeochem. Cycles 2008, 22, 1–13. [Google Scholar] [CrossRef]
- Holmes, C.D.; Jacob, D.J.; Corbitt, E.S.; Mao, J.; Yang, X.; Talbot, R.; Slemr, F. Global atmospheric model for mercury including oxidation by bromine atoms. Atmos. Chem. Phys. 2010, 10, 12037–12057. [Google Scholar] [CrossRef] [Green Version]
- Carlson, R.W. The Mantle and Core: Treatise on Geochemistry; Elsevier: Amsterdam, The Netherlands, 2005; Volume 2, ISBN 0080549012. [Google Scholar]
- Flanagan, F.J.; Moore, R.; Aruscavage, P.J. Mercury in Geologic Reference Samples. Geostand. Newsl. 1982, 6, 25–46. [Google Scholar] [CrossRef]
- Peña-Rodríguez, S.; Pontevedra-Pombal, X.; Fernández-Calviño, D.; Taboada, T.; Arias-Estévez, M.; Martínez-Cortizas, A.; Nóvoa-Muñoz, J.C.; García-Rodeja, E. Mercury content in volcanic soils across Europe and its relationship with soil properties. J. Soils Sediments 2012, 12, 542–555. [Google Scholar] [CrossRef]
- Roulet, M.; Lucotte, M.; Saint-Aubin, A.; Tran, S.; Rheault, I.; Farella, N.; Dezencourt, J.; Passos, C.-J.S.; Soares, G.S.; Guimaraes, J.-R. The geochemistry of mercury in central Amazonian soils developed on the Alter-do-Chao formation of the lower Tapajos River Valley, Para state, Brazil. Sci. Total Environ. 1998, 223, 1–24. [Google Scholar] [CrossRef]
- García-Rodeja, E.; Nóvoa, J.C.; Pontevedra, X.; Martínez-Cortizas, A.; Buurman, P. Aluminium fractionation of European volcanic soils by selective dissolution techniques. Soils Volcan. Reg. Eur. 2004, 56, 325–351. [Google Scholar] [CrossRef]
- Nóvoa-Muñoz, J.C.; Pontevedra-Pombal, X.; Martínez-Cortizas, A.; García-Rodeja Gayoso, E. Mercury accumulation in upland acid forest ecosystems nearby a coal-fired power-plant in Southwest Europe (Galicia, NW Spain). Sci. Total Environ. 2008, 394, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Rydberg, J.; Karlsson, J.; Nyman, R.; Wanhatalo, I.; Näthe, K.; Bindler, R. Importance of vegetation type for mercury sequestration in the northern Swedish mire, Rödmossamyran. Geochim. Cosmochim. Acta 2010, 74, 7116–7126. [Google Scholar] [CrossRef]
- Margalef, O. The Last 70 Ky of Rano Aroi (Easter Island, 27 °S) Peat Record: New Insights for the Central Pacific Paleoclimatology; Universitat de Barcelona: Barcelona, Spain, 2014. [Google Scholar]
- Martínez Cortizas, A.; Biester, H.; Mighall, T.; Bindler, R. Climate-driven enrichment of pollutants in peatlands. Biogeosciences 2007, 4, 905–911. [Google Scholar] [CrossRef]
- Hesse, P.P.; McTainsh, G.H. Last Glacial Maximum to Early Holocene Wind Strength in the Mid-latitudes of the Southern Hemisphere from Aeolian Dust in the Tasman Sea. Quat. Res. 1999, 52, 343–349. [Google Scholar] [CrossRef]
- Cannon, W.F.; Dean, W.E.; Bullock, J.H. Effects of Holocene Climate Change on Mercury Deposition in Elk Lake, Minnesota: The Importance of Eolian Transport in the Mercury Cycle Effects of Holocene climate change on mercury deposition in Elk Lake, Minnesota: The importance of eolian transpor. Geology 2003, 31, 187–190. [Google Scholar] [CrossRef]
- DeMenocal, P.B. Cultural Responses to Climate Change During the Late Holocene. Science 2001, 292, 667–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.P.; Fitzgerald, W.F. Sea-Air Partitioning of Mercury in the Equatorial Pacific Ocean. Science 1986, 231, 1131–1133. [Google Scholar] [CrossRef] [PubMed]
- Vandal, G.M.; Fitzgerald, W.F.; Boutron, C.F.; Candelone, J.-P. Variations in mercury deposition to Antarctica over the past 34,000 years. Nature 1993, 362, 621–623. [Google Scholar] [CrossRef]
- Salvatteci, R.; Gutierrez, D.; Sifeddine, A.; Ortlieb, L.; Druffel, E.; Boussafir, M.; Schneider, R. Centennial to millennial-scale changes in oxygenation and productivity in the Eastern Tropical South Pacific during the last 25,000 years. Q. Sci. Rev. 2016, 131, 102–117. [Google Scholar] [CrossRef]
- Costa, K.M.; Jacobel, A.W.; McManus, J.F.; Anderson, R.F.; Winckler, G.; Thiagarajan, N. Productivity patterns in the Equatorial Pacific over the last 30,000 years. Glob. Biogeochem. Cycles 2017, 31, 1–16. [Google Scholar] [CrossRef]
- Bagnato, E.; Aiuppa, A.; Parello, F.; Calabrese, S.; D’Alessandro, W.; Mather, T.A.; McGonigle, A.J.S.; Pyle, D.M.; Wängberg, I. Degassing of gaseous (elemental and reactive) and particulate mercury from Mount Etna volcano (Southern Italy). Atmos. Environ. 2007, 41, 7377–7388. [Google Scholar] [CrossRef]
- Witt, M.L.I.; Mather, T.A.; Pyle, D.M.; Aiuppa, A.; Bagnato, E.; Tsanev, V.I. Mercury and halogen emissions from Masaya and Telica volcanoes, Nicaragua. J. Geophys. Res. 2008, 113, B6. [Google Scholar] [CrossRef]
- Von Glasow, R. Atmospheric chemistry in volcanic plumes. Proc. Natl. Acad. Sci. USA 2010, 107, 6594–6599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, P.E. Preliminary account of recent geological investigations on Easter Island. Geol. Mag. 1967, 104, 116–122. [Google Scholar] [CrossRef]
- McCulloch, R.D.; Davies, S.J. Late-glacial and Holocene palaeoenvironmental change in the central Strait of Magellan, southern Patagonia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2001, 173, 143–173. [Google Scholar] [CrossRef]
- Stern, C.R. Holocene tephrochronology record of large explosive eruptions in the southernmost Patagonian Andes. Bull. Volcanol. 2008, 70, 435–454. [Google Scholar] [CrossRef]
- Vanneste, H.; De Vleeschouwer, F.; Bertrand, S.; Martínez-Cortizas, A.; Vanderstraeten, A.; Mattielli, N.; Coronato, A.; Piotrowska, N.; Jeandel, C.; Roux, G. Le Elevated dust deposition in Tierra del Fuego (Chile) resulting from Neoglacial Darwin Cordillera glacier fluctuations. J. Quat. Sci. 2016, 31, 713–722. [Google Scholar] [CrossRef]
- De Simone, F.; Cinnirella, S.; Gencarelli, C.N.; Yang, X.; Hedgecock, I.M.; Pirrone, N. Model study of global mercury deposition from biomass burning. Environ. Sci. Technol. 2015, 49, 6712–6721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Simone, F.; Artaxo, P.; Bencardino, M.; Cinnirella, S.; Carbone, F.; D’Amore, F.; Dommergue, A.; Bin Feng, X.; Gencarelli, C.N.; Hedgecock, I.M.; et al. Particulate-phase mercury emissions from biomass burning and impact on resulting deposition: A modelling assessment. Atmos. Chem. Phys. 2017, 17, 1881–1899. [Google Scholar] [CrossRef] [PubMed]
- Power, M.J.; Marlon, J.; Ortiz, N.; Bartlein, P.J.; Harrison, S.P.; Mayle, F.E.; Ballouche, A.; Bradshaw, R.H.W.; Carcaillet, C.; Cordova, C.; et al. Changes in fire regimes since the last glacial maximum: An assessment based on a global synthesis and analysis of charcoal data. Clim. Dyn. 2008, 30, 887–907. [Google Scholar] [CrossRef]






| Local/English Name | Scientific Name | Hg (ng g−1) |
|---|---|---|
| Kikuyu/Kikuyu grass | Pennisetum clandestinum | 1945 |
| Nga’atu/Totora (duster) | Scirpus californicus (duster) | 1804 |
| Nga’atu/Totora (rhizome) | Scirpus californicus (rhizome) | 341 |
| /Rush | no identified | 11,175 |
| Maku Piro/molassesgrass | Melinis minutiflora | 1470 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Rodríguez, M.; Margalef, O.; Corella, J.P.; Saiz-Lopez, A.; Pla-Rabes, S.; Giralt, S.; Martínez Cortizas, A. The Role of Climate: 71 ka of Atmospheric Mercury Deposition in the Southern Hemisphere Recorded by Rano Aroi Mire, Easter Island (Chile). Geosciences 2018, 8, 374. https://doi.org/10.3390/geosciences8100374
Pérez-Rodríguez M, Margalef O, Corella JP, Saiz-Lopez A, Pla-Rabes S, Giralt S, Martínez Cortizas A. The Role of Climate: 71 ka of Atmospheric Mercury Deposition in the Southern Hemisphere Recorded by Rano Aroi Mire, Easter Island (Chile). Geosciences. 2018; 8(10):374. https://doi.org/10.3390/geosciences8100374
Chicago/Turabian StylePérez-Rodríguez, Marta, Olga Margalef, Juan Pablo Corella, Alfonso Saiz-Lopez, Sergi Pla-Rabes, Santiago Giralt, and Antonio Martínez Cortizas. 2018. "The Role of Climate: 71 ka of Atmospheric Mercury Deposition in the Southern Hemisphere Recorded by Rano Aroi Mire, Easter Island (Chile)" Geosciences 8, no. 10: 374. https://doi.org/10.3390/geosciences8100374
APA StylePérez-Rodríguez, M., Margalef, O., Corella, J. P., Saiz-Lopez, A., Pla-Rabes, S., Giralt, S., & Martínez Cortizas, A. (2018). The Role of Climate: 71 ka of Atmospheric Mercury Deposition in the Southern Hemisphere Recorded by Rano Aroi Mire, Easter Island (Chile). Geosciences, 8(10), 374. https://doi.org/10.3390/geosciences8100374