Regulation of Polyamine Metabolism by Curcumin for Cancer Prevention and Therapy
Abstract
:1. Introduction
2. Polyamines and Cancer
2.1. Targeting Polyamine Metabolism for Cancer Prevention
2.2. Targeting Polyamine Metabolism for Cancer Treatment
3. Dietary Factors with Potential to Moderate Carcinogenesis through the Polyamine Pathway
3.1. Plant Polyphenols
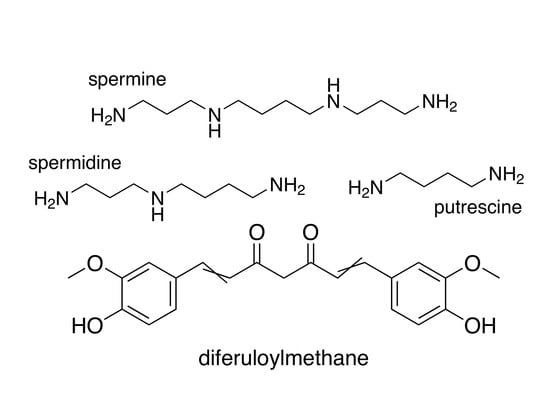
3.2. Curcumin
4. Investigations into the Antitumor Potential of Curcumin through Modulating Polyamine Metabolism
4.1. Evidence of the Chemopreventive Activity of Curcumin in Carcinogenesis Models
4.1.1. Topical Application of Curcumin in Animal Models of Skin Cancer
4.1.2. Dietary Curcumin in Rodent Models of Carcinogenesis
4.2. Investigations into Polyamine-Associated Effects of Curcumin on Established Tumors—Potential for Cancer Treatment
5. Translational Potential, Clinical Trials, and Limitations
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, Y.; Antony, S.; Meitzler, J.L.; Doroshow, J.H. Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett. 2014, 345, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Okada, F. Inflammation-related carcinogenesis: Current findings in epidemiological trends, causes and mechanisms. Yonago Acta Med. 2014, 57, 65–72. [Google Scholar] [PubMed]
- Pegg, A.E. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988, 48, 759–774. [Google Scholar] [PubMed]
- Gerner, E.W.; Meyskens, F.L., Jr. Polyamines and cancer: Old molecules, new understanding. Nat. Rev. Cancer 2004, 4, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.J.; Tajmir-Riahi, H.A.; Thomas, T. Polyamine-DNA interactions and development of gene delivery vehicles. Amino Acids 2016, 48, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Lentini, A.; Abbruzzese, A.; Caraglia, M.; Marra, M.; Beninati, S. Protein-polyamine conjugation by transglutaminase in cancer cell differentiation: Review article. Amino Acids 2004, 26, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Baronas, V.A.; Kurata, H.T. Inward rectifiers and their regulation by endogenous polyamines. Front. Physiol. 2014, 5, 325. [Google Scholar] [CrossRef] [PubMed]
- Williams, K. Modulation and block of ion channels: A new biology of polyamines. Cell. Signal. 1997, 9, 1–13. [Google Scholar] [CrossRef]
- Thomas, T.; Thomas, T.J. Polyamine metabolism and cancer. J. Cell. Mol. Med. 2003, 7, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Shantz, L.M.; Levin, V.A. Regulation of ornithine decarboxylase during oncogenic transformation: Mechanisms and therapeutic potential. Amino Acids 2007, 33, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A.; Pegg, A.E. Polyamine catabolism and disease. Biochem. J. 2009, 421, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.R.; Challa, A.; Gupta, S.; Bostwick, D.G.; Ahmad, N.; Agarwal, R.; Marengo, S.R.; Amini, S.B.; Paras, F.; MacLennan, G.T.; et al. Overexpression of ornithine decarboxylase in prostate cancer and prostatic fluid in humans. Clin. Cancer Res. 1999, 5, 143–147. [Google Scholar] [PubMed]
- Wallace, H.M.; Caslake, R. Polyamines and colon cancer. Eur. J. Gastroenterol. Hepatol. 2001, 13, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Thomas, T.J. Polyamines in cell growth and cell death: Molecular mechanisms and therapeutic applications. Cell. Mol. Life Sci. 2001, 58, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, S.K. Polyamines and nonmelanoma skin cancer. Toxicol. Appl. Pharmacol. 2007, 224, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Manni, A.; Mauger, D.; Gimotty, P.; Badger, B. Prognostic influence on survival of increased ornithine decarboxylase activity in human breast cancer. Clin. Cancer Res. 1996, 2, 1901–1906. [Google Scholar] [PubMed]
- Hibshoosh, H.; Johnson, M.; Weinstein, I.B. Effects of overexpression of ornithine decarboxylase (ODC) on growth control and oncogene-induced cell transformation. Oncogene 1991, 6, 739–743. [Google Scholar] [PubMed]
- Auvinen, M.; Paasinen, A.; Andersson, L.C.; Holtta, E. Ornithine decarboxylase activity is critical for cell transformation. Nature 1992, 360, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.K.; Trempus, C.S.; Gilmour, S.K. Co-operation between follicular ornithine decarboxylase and v-Ha-ras induces spontaneous papillomas and malignant conversion in transgenic skin. Carcinogenesis 1998, 19, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.J.; Meyers, C.; Laimins, L.A.; Hay, N. c-Myc induces the expression and activity of ornithine decarboxylase. Cell. Growth Differ. 1993, 4, 879–883. [Google Scholar] [PubMed]
- Bello-Fernandez, C.; Packham, G.; Cleveland, J.L. The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc. Natl Acad. Sci. USA 1993, 90, 7804–7808. [Google Scholar] [CrossRef] [PubMed]
- Forshell, T.P.; Rimpi, S.; Nilsson, J.A. Chemoprevention of B-cell lymphomas by inhibition of the Myc target spermidine synthase. Cancer Prev. Res. (Phila) 2010, 3, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Holtta, E.; Sistonen, L.; Alitalo, K. The mechanisms of ornithine decarboxylase deregulation in c-Ha-ras oncogene-transformed NIH 3T3 cells. J. Biol. Chem. 1988, 263, 4500–4507. [Google Scholar] [PubMed]
- Shantz, L.M. Transcriptional and translational control of ornithine decarboxylase during Ras transformation. Biochem. J. 2004, 377, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Shantz, L.M.; Pegg, A.E. Ornithine decarboxylase induction in transformation by H-Ras and RhoA. Cancer Res. 1998, 58, 2748–2753. [Google Scholar] [PubMed]
- Linsalata, M.; Notarnicola, M.; Caruso, M.G.; Di Leo, A.; Guerra, V.; Russo, F. Polyamine biosynthesis in relation to K-ras and p-53 mutations in colorectal carcinoma. Scand. J. Gastroenterol. 2004, 39, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.A.; Keller, U.B.; Baudino, T.A.; Yang, C.; Norton, S.; Old, J.A.; Nilsson, L.M.; Neale, G.; Kramer, D.L.; Porter, C.W.; et al. Targeting ornithine decarboxylase in Myc-induced lymphomagenesis prevents tumor formation. Cancer Cell. 2005, 7, 433–444. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.G.; Megosh, L.C.; Gilliard, G.; Soler, A.P. Ornithine decarboxylase overexpression is a sufficient condition for tumor promotion in mouse skin. Cancer Res. 1997, 57, 2630–2637. [Google Scholar] [PubMed]
- Gilmour, S.K.; Robertson, F.M.; Megosh, L.; O’Connell, S.M.; Mitchell, J.; O’Brien, T.G. Induction of ornithine decarboxylase in specific subpopulations of murine epidermal cells following multiple exposures to 12-O-tetradecanoylphorbol-13-acetate, mezerein and ethyl phenylpropriolate. Carcinogenesis 1992, 13, 51–56. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.G.; Simsiman, R.C.; Boutwell, R.K. Induction of the polyamine-biosynthetic enzymes in mouse epidermis by tumor-promoting agents. Cancer Res. 1975, 35, 1662–1670. [Google Scholar] [PubMed]
- Meyskens, F.L., Jr.; Gerner, E.W. Development of difluoromethylornithine (DFMO) as a chemoprevention agent. Clin. Cancer Res. 1999, 5, 945–951. [Google Scholar] [PubMed]
- Russell, D.; Snyder, S.H. Amine synthesis in rapidly growing tissues: Ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc. Natl. Acad. Sci. USA 1968, 60, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A., Jr.; Marton, L.J. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat. Rev. Drug Discov. 2007, 6, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Murray-Stewart, T.R.; Woster, P.M.; Casero, R.A., Jr. Targeting polyamine metabolism for cancer therapy and prevention. Biochem. J. 2016, 473, 2937–2953. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, B.W.; Bey, P.; Danzin, C.; Jung, M.J.; Casara, P.; Vevert, J.P. Catalytic irreversible inhibition of mammalian ornithine decarboxylase (E.C.4.1.1.17) by substrate and product analogs. J. Am. Chem. Soc. 1978, 100, 2551–2553. [Google Scholar] [CrossRef]
- McCann, P.P.; Pegg, A.E. Ornithine decarboxylase as an enzyme target for therapy. Pharmacol. Ther. 1992, 54, 195–215. [Google Scholar] [CrossRef]
- Meyskens, F.L., Jr.; Gerner, E.W.; Emerson, S.; Pelot, D.; Durbin, T.; Doyle, K.; Lagerberg, W. Effect of α-difluoromethylornithine on rectal mucosal levels of polyamines in a randomized, double-blinded trial for colon cancer prevention. J. Natl. Cancer Inst. 1998, 90, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Gerner, E.W.; Meyskens, F.L., Jr. Combination chemoprevention for colon cancer targeting polyamine synthesis and inflammation. Clin. Cancer Res. 2009, 15, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Ignatenko, N.A.; Besselsen, D.G.; Stringer, D.E.; Blohm-Mangone, K.A.; Cui, H.; Gerner, E.W. Combination chemoprevention of intestinal carcinogenesis in a murine model of familial adenomatous polyposis. Nutr. Cancer 2008, 60, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, R.F.; Cole, C.E.; Tutsch, K.; Newton, M.A.; Kelloff, G.; Hawk, E.T.; Lubet, R.A. Chemopreventive efficacy of combined piroxicam and difluoromethylornithine treatment of Apc mutant Min mouse adenomas, and selective toxicity against Apc mutant embryos. Cancer Res. 2000, 60, 1864–1870. [Google Scholar] [PubMed]
- Meyskens, F.L., Jr.; McLaren, C.E.; Pelot, D.; Fujikawa-Brooks, S.; Carpenter, P.M.; Hawk, E.; Kelloff, G.; Lawson, M.J.; Kidao, J.; McCracken, J.; et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: A randomized placebo-controlled, double-blind trial. Cancer Prev. Res. (Phila) 2008, 1, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Zell, J.A.; Pelot, D.; Chen, W.P.; McLaren, C.E.; Gerner, E.W.; Meyskens, F.L. Risk of cardiovascular events in a randomized placebo-controlled, double-blind trial of difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas. Cancer Prev. Res. (Phila) 2009, 2, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Nowotarski, S.L.; Woster, P.M.; Casero, R.A., Jr. Polyamines and cancer: Implications for chemotherapy and chemoprevention. Expert Rev. Mol. Med. 2013, 15, e3. [Google Scholar] [CrossRef] [PubMed]
- Tsioulias, G.J.; Go, M.F.; Rigas, B. NSAIDs and colorectal cancer control: Promise and challenges. Curr. Pharmacol. Rep. 2015, 1, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Schechter, P.J.; Barlow, J.L.R.; Sjoerdsma, A. Clinical aspects of inhibition of ornithine decarboxylase with emphasis on therapeutic trials of eflornithine (DFMO) in cancer and protozoan diseases. In Inhibition of Polyamine Metabolism. Biological Significance and Basis for New Therapies; McCann, P.P., Pegg, A.E., Sjoerdsma, A., Eds.; Academic Press: Orlando, FL, USA, 1987; pp. 345–364. [Google Scholar]
- Seiler, N. Thirty years of polyamine-related approaches to cancer therapy. Retrospect and prospect. Part 1. Selective enzyme inhibitors. Curr. Drug Targets 2003, 4, 537–564. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, V.; DeStefano Shields, C.; Murray-Stewart, T.; Casero, R.A., Jr. Polyamine catabolism in carcinogenesis: Potential targets for chemotherapy and chemoprevention. Amino Acids 2014, 46, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A., Jr.; Wang, Y.; Stewart, T.M.; Devereux, W.; Hacker, A.; Wang, Y.; Smith, R.; Woster, P.M. The role of polyamine catabolism in anti-tumour drug response. Biochem. Soc. Trans. 2003, 31, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Murray-Stewart, T.; Casero, R., Jr. Mammalian polyamine catabolism. In Polyamines; Kusano, T., Suzuki, H., Eds.; Springer: Tokyo, Japan, 2015; pp. 61–75. [Google Scholar]
- Reddy, V.K.; Valasinas, A.; Sarkar, A.; Basu, H.S.; Marton, L.J.; Frydman, B. Conformationally restricted analogues of 1N,12N-bisethylspermine: Synthesis and growth inhibitory effects on human tumor cell lines. J. Med. Chem. 1998, 41, 4723–4732. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Murray-Stewart, T.; Wang, Y.; Yu, F.; Li, J.; Marton, L.J.; Casero, R.A., Jr.; Oupicky, D. Self-immolative nanoparticles for simultaneous delivery of microRNA and targeting of polyamine metabolism in combination cancer therapy. J. Control. Release 2017, 246, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Murray-Stewart, T.; Ferrari, E.; Xie, Y.; Yu, F.; Marton, L.J.; Oupicky, D.; Casero, R.A., Jr. Biochemical evaluation of the anticancer potential of the polyamine-based nanocarrier Nano11047. PLoS ONE 2017, 12, e0175917. [Google Scholar] [CrossRef]
- Linsalata, M.; Orlando, A.; Russo, F. Pharmacological and dietary agents for colorectal cancer chemoprevention: Effects on polyamine metabolism (review). Int. J. Oncol. 2014, 45, 1802–1812. [Google Scholar] [CrossRef] [PubMed]
- Linsalata, M.; Russo, F. Nutritional factors and polyamine metabolism in colorectal cancer. Nutrition 2008, 24, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The essential medicinal chemistry of curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Amin, A.R.; Chen, Z.G.; Shin, D.M. New perspectives of curcumin in cancer prevention. Cancer Prev. Res. (Phila) 2013, 6, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Mittal, S.; Sak, K.; Tuli, H.S. Molecular mechanisms underlying chemopreventive potential of curcumin: Current challenges and future perspectives. Life Sci. 2016, 148, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Aggarwal, B.B. Activation of transcription factor NF-κB is suppressed by curcumin (diferuloylmethane) [corrected]. J. Biol. Chem. 1995, 270, 24995–25000. [Google Scholar] [CrossRef] [PubMed]
- Jobin, C.; Bradham, C.A.; Russo, M.P.; Juma, B.; Narula, A.S.; Brenner, D.A.; Sartor, R.B. Curcumin blocks cytokine-mediated NF-κB activation and proinflammatory gene expression by inhibiting inhibitory factor I-κB kinase activity. J. Immunol. 1999, 163, 3474–3483. [Google Scholar] [PubMed]
- O’Brien, T.G. The induction of ornithine decarboxylase as an early, possibly obligatory, event in mouse skin carcinogenesis. Cancer Res. 1976, 36, 2644–2653. [Google Scholar] [PubMed]
- Huang, M.T.; Smart, R.C.; Wong, C.Q.; Conney, A.H. Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and ferulic acid on tumor promotion in mouse skin by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1988, 48, 5941–5946. [Google Scholar] [PubMed]
- Lu, Y.P.; Chang, R.L.; Huang, M.T.; Conney, A.H. Inhibitory effect of curcumin on 12-O-tetradecanoylphorbol-13-acetate-induced increase in ornithine decarboxylase mRNA in mouse epidermis. Carcinogenesis 1993, 14, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Strickland, J.E.; Greenhalgh, D.A.; Koceva-Chyla, A.; Hennings, H.; Restrepo, C.; Balaschak, M.; Yuspa, S.H. Development of murine epidermal cell lines which contain an activated rasHa oncogene and form papillomas in skin grafts on athymic nude mouse hosts. Cancer Res. 1988, 48, 165–169. [Google Scholar] [PubMed]
- Lee, S.K.; Pezzuto, J.M. Evaluation of the potential of cancer chemopreventive activity mediated by inhibition of 12-O-tetradecanoyl phorbol 13-acetate-induced ornithine decarboxylase activity. Arch. Pharm. Res. 1999, 22, 559–564. [Google Scholar] [CrossRef] [PubMed]
- White, E.L.; Ross, L.J.; Schmid, S.M.; Kelloff, G.J.; Steele, V.E.; Hill, D.L. Screening of potential cancer-preventing chemicals for inhibition of induction of ornithine decarboxylase in epithelial cells from rat trachea. Oncol. Rep. 1998, 5, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Ramchandani, A.G.; Maru, G.B. Curcumin decreases 12-O-tetradecanoylphorbol-13-acetate-induced protein kinase C translocation to modulate downstream targets in mouse skin. Carcinogenesis 2008, 29, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, C.; Oguro, T.; Yoshida, T.; Wen, C.Q.; Sueki, H.; Iijima, M. Enhancing effect of ultraviolet A on ornithine decarboxylase induction and dermatitis evoked by 12-O-tetradecanoylphorbol-13-acetate and its inhibition by curcumin in mouse skin. Dermatology 1996, 193, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Oguro, T.; Yoshida, T. Effect of ultraviolet A on ornithine decarboxylase and metallothionein gene expression in mouse skin. Photodermatol. Photoimmunol. Photomed. 2001, 17, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, X.F. The signal pathways in azoxymethane-induced colon cancer and preventive implications. Cancer Biol. Ther. 2009, 8, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.V.; Simi, B.; Reddy, B.S. Inhibition by dietary curcumin of azoxymethane-induced ornithine decarboxylase, tyrosine protein kinase, arachidonic acid metabolism and aberrant crypt foci formation in the rat colon. Carcinogenesis 1993, 14, 2219–2225. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ishigamori, R. Understanding carcinogenesis for fighting oral cancer. J. Oncol. 2011, 2011, 603740. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Makita, H.; Ohnishi, M.; Hirose, Y.; Wang, A.; Mori, H.; Satoh, K.; Hara, A.; Ogawa, H. Chemoprevention of 4-nitroquinoline 1-oxide-induced oral carcinogenesis by dietary curcumin and hesperidin: Comparison with the protective effect of β-carotene. Cancer Res. 1994, 54, 4653–4659. [Google Scholar] [PubMed]
- Tanaka, T.; Kojima, T.; Hara, A.; Sawada, H.; Mori, H. Chemoprevention of oral carcinogenesis by DL-α-difluoromethylornithine, an ornithine decarboxylase inhibitor: Dose-dependent reduction in 4-nitroquinoline 1-oxide-induced tongue neoplasms in rats. Cancer Res. 1993, 53, 772–776. [Google Scholar] [PubMed]
- Okazaki, Y.; Iqbal, M.; Okada, S. Suppressive effects of dietary curcumin on the increased activity of renal ornithine decarboxylase in mice treated with a renal carcinogen, ferric nitrilotriacetate. Biochim. Biophys. Acta 2005, 1740, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Syng-Ai, C.; Kumari, A.L.; Khar, A. Effect of curcumin on normal and tumor cells: Role of glutathione and Bcl-2. Mol. Cancer Ther. 2004, 3, 1101–1108. [Google Scholar] [PubMed]
- Fang, J.; Lu, J.; Holmgren, A. Thioredoxin reductase is irreversibly modified by curcumin: A novel molecular mechanism for its anticancer activity. J. Biol. Chem. 2005, 280, 25284–25290. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, A.; Barik, A.; Mishra, B.; Rathinasamy, K.; Pandey, R.; Priyadarsini, K.I. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim. Biophys. Acta 2008, 1780, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Cao, Y.; Greten, F.R.; Li, Z.W. NF-κB in cancer: From innocent bystander to major culprit. Nat. Rev. Cancer 2002, 2, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Tacchini, L.; De Ponti, C.; Matteucci, E.; Follis, R.; Desiderio, M.A. Hepatocyte growth factor-activated NF-κB regulates HIF-1 activity and ODC expression, implicated in survival, differently in different carcinoma cell lines. Carcinogenesis 2004, 25, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Babbar, N.; Gerner, E.W.; Casero, R.A., Jr. Induction of spermidine/spermine N1-acetyltransferase (SSAT) by aspirin in Caco-2 colon cancer cells. Biochem. J. 2006, 394, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Thomas, T.; Shirahata, A.; Sigal, L.H.; Thomas, T.J. Activation of nuclear factor κB by polyamines in breast cancer cells. Biochemistry 1999, 38, 14763–14774. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.; Pantazis, P.; McQueen, T.; Aggarwal, B.B. Antiproliferative effect of curcumin (diferuloylmethane) against human breast tumor cell lines. Anticancer Drugs 1997, 8, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.J.; Santhakumaran, L.M.; Parikh, M.S.; Thomas, T. A possible mechanism for the growth inhibitory action of curcumin on HER-2 over expressing SK-BR-3 breast cancer cells involves the polyamine pathway. Cancer Res. 2004, 64, 168–169. [Google Scholar]
- Berrak, O.; Akkoc, Y.; Arisan, E.D.; Coker-Gurkan, A.; Obakan-Yerlikaya, P.; Palavan-Unsal, N. The inhibition of PI3K and NFκB promoted curcumin-induced cell cycle arrest at G2/M via altering polyamine metabolism in Bcl-2 overexpressing MCF-7 breast cancer cells. Biomed. Pharmacother. 2016, 77, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Quinn, B.A.; Das, S.K.; Dash, R.; Emdad, L.; Dasgupta, S.; Wang, X.Y.; Dent, P.; Reed, J.C.; Pellecchia, M.; et al. Targeting the Bcl-2 family for cancer therapy. Expert Opin. Ther. Targets 2013, 17, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Ricca, A.; Biroccio, A.; Del Bufalo, D.; Mackay, A.R.; Santoni, A.; Cippitelli, M. Bcl-2 over-expression enhances NF-κB activity and induces MMP-9 transcription in human MCF7ADR breast-cancer cells. Int. J. Cancer 2000, 86, 188–196. [Google Scholar] [CrossRef]
- Liao, Y.F.; Hung, H.C.; Hour, T.C.; Hsu, P.C.; Kao, M.C.; Tsay, G.J.; Liu, G.Y. Curcumin induces apoptosis through an ornithine decarboxylase-dependent pathway in human promyelocytic leukemia HL-60 cells. Life Sci. 2008, 82, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Luk, G.D.; Baylin, S.B. Ornithine decarboxylase as a biologic marker in familial colonic polyposis. N. Engl. J. Med. 1984, 311, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Giardiello, F.M.; Hamilton, S.R.; Hylind, L.M.; Yang, V.W.; Tamez, P.; Casero, R.A., Jr. Ornithine decarboxylase and polyamines in familial adenomatous polyposis. Cancer Res. 1997, 57, 199–201. [Google Scholar] [PubMed]
- Perkins, S.; Verschoyle, R.D.; Hill, K.; Parveen, I.; Threadgill, M.D.; Sharma, R.A.; Williams, M.L.; Steward, W.P.; Gescher, A.J. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol. Biomark. Prev. 2002, 11, 535–540. [Google Scholar]
- Cruz-Correa, M.; Shoskes, D.A.; Sanchez, P.; Zhao, R.; Hylind, L.M.; Wexner, S.D.; Giardiello, F.M. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin. Gastroenterol. Hepatol. 2006, 4, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Adiwidjaja, J.; McLachlan, A.J.; Boddy, A.V. Curcumin as a clinically-promising anti-cancer agent: Pharmacokinetics and drug interactions. Expert Opin. Drug Metab. Toxicol. 2017, 13, 953–972. [Google Scholar] [CrossRef] [PubMed]
- Padhye, S.; Chavan, D.; Pandey, S.; Deshpande, J.; Swamy, K.V.; Sarkar, F.H. Perspectives on chemopreventive and therapeutic potential of curcumin analogs in medicinal chemistry. Mini Rev. Med. Chem. 2010, 10, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Pati, H.N.; Das, U.; Quail, J.W.; Kawase, M.; Sakagami, H.; Dimmock, J.R. Cytotoxic 3,5-bis(benzylidene)piperidin-4-ones and N-acyl analogs displaying selective toxicity for malignant cells. Eur. J. Med. Chem. 2008, 43, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Youssef, K.M.; El-Sherbeny, M.A.; El-Shafie, F.S.; Farag, H.A.; Al-Deeb, O.A.; Awadalla, S.A. Synthesis of curcumin analogues as potential antioxidant, cancer chemopreventive agents. Arch. Pharm. (Weinheim) 2004, 337, 42–54. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murray-Stewart, T.; Casero, R.A. Regulation of Polyamine Metabolism by Curcumin for Cancer Prevention and Therapy. Med. Sci. 2017, 5, 38. https://doi.org/10.3390/medsci5040038
Murray-Stewart T, Casero RA. Regulation of Polyamine Metabolism by Curcumin for Cancer Prevention and Therapy. Medical Sciences. 2017; 5(4):38. https://doi.org/10.3390/medsci5040038
Chicago/Turabian StyleMurray-Stewart, Tracy, and Robert A. Casero. 2017. "Regulation of Polyamine Metabolism by Curcumin for Cancer Prevention and Therapy" Medical Sciences 5, no. 4: 38. https://doi.org/10.3390/medsci5040038
APA StyleMurray-Stewart, T., & Casero, R. A. (2017). Regulation of Polyamine Metabolism by Curcumin for Cancer Prevention and Therapy. Medical Sciences, 5(4), 38. https://doi.org/10.3390/medsci5040038