Environment Friendly Pretreatment Approaches for the Bioconversion of Lignocellulosic Biomass into Biofuels and Value-Added Products
Abstract
:1. Introduction
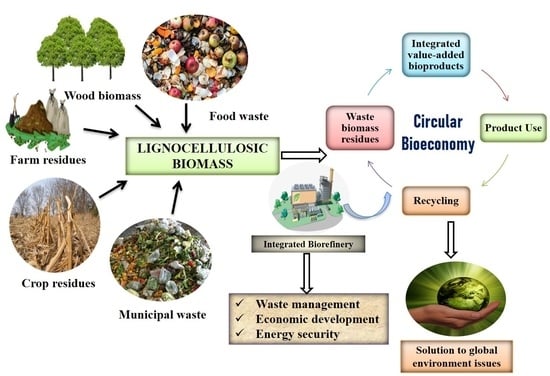
2. Lignocellulosic Biorefinery Concept for Circular Bioeconomy
- a.
- Phase I biorefinery (one feed, one preset processing technology, and one main product). For example, in the European Union, vegetable oil biorefinery involves the production of biodiesel by the transesterification of rapeseed oil;
- b.
- Phase II biorefinery (one feed, multiple processing technologies, and multiple end products). For example, in Sweden, forestry feedstock is refined to produce cellulose, lignosulphonate, and bioethanol;
- c.
- Phase III biorefinery (multiple feedstocks, multiple processing technologies, and multiple end products). For example, lignocellulosic biomass-based biorefinery, two-platform biorefinery, green biorefinery, and others.
3. Lignocellulose Composition and Structure
3.1. Cellulose
3.2. Hemicellulose
3.3. Lignin
3.4. Other Components
4. Recent Advances in Pretreatment Technologies for Lignocellulosic Biomass
4.1. Physical Pretreatment
4.1.1. Ultrasound Pretreatment
4.1.2. Microwave Irradiation
4.2. Chemical Pretreatment
4.2.1. Ionic Liquids (ILs)
4.2.2. Deep Eutectic Solvents (DESs)
4.2.3. Organosolv Pretreatment
4.3. Physicochemical Pretreatment
4.4. Biological Pretreatment
| Pretreatment Process | Merit | Demerit | Biomass/Pretreatment Conditions | Significant Results | Reference |
|---|---|---|---|---|---|
| Acid treatment | Hemicellulose hydrolysis, increased biomass porosity | Formation of furfurals, hydroxymethyl furfural, corrosion | Sugarcane bagasse/4.95% phosphoric acid, 80 °C, 375 min) | 98% glucose yield, 99% saccharification efficiency. | [73] |
| γ-Valerolactone/dilute H2SO4 (4:1, v/v), 120 °C, 60 min. | 89.1% glucose yield | [96] | |||
| Alkali pretreatment | High lignin removal, hemicellulose hydrolysis | Formation of magnesium and calcium salts, long residence time | Date palm/20 % NH3, 80 °C, 12 h | High biochemical methane potential (309.76 mL CH4/g-TS) | [74] |
| Giant reed biomass/20% NaOH | High glucose yield (44.9%), high H2 yield (98.3 mL/g TS) | [97] | |||
| Ionic liquid pretreatment | Liquid at room temperature, low toxicity, low vapor pressure, high digestibility, thermal stability | Expensive and toxic to hydrolytic enzymes | Almond wood/ethanolamine acetate (15 wt % solid loading) | High glucose (24–82%) and xylose yields (14–80%); 60.8% fermentation efficiency | [98] |
| Stinging nettle stems/1-butyl-3-methylimidazolium acetate (10 g biomass in 50 cm3 IL, 120 °C, 2 h) | High ethanol concentration (7.3 g L−1) | [99] | |||
| Deep eutectic solvent pretreatment | Easy synthesis, low-cost, biodegradable less toxic, recyclable | High viscosity, hygroscopic | Parthenium hysterophorus/ChCl/sorbitol (1:5), | Higher sugar yield (148.54 mg/g biomass) | [16] |
| Sugarcane bagasse/ChCl:glycerol (1:10)-ultrasound | Higher reducing sugar titer (276.8mg/g substrate) | [9] | |||
| Banana peel waste/ChCl-based DES | High total reducing sugar yield of 72.9% | [100] | |||
| Organosolv pretreatment | Easy recovery and recycling, effective delignification | Repeated washings of pretreated LCB, expensive solvents | Pine, beechwood/mild oxidative (acetone, tetrahydrofuran, and ethanol) | High lactic acid production (beechwood: 62 g L−1; pine: 36.4 g L−1) | [85] |
| Corn stover/aqueous ethanol (60%, v/v), n-propylamine (10 mmol/g, biomass) | High sugar yield (83.2%) and delignification (83.2%) | [87] | |||
| Ultrasound pretreatment | Size reduction, proper mixing of biomass with solvent, disintegration of cell wall components, less process time, assistive technique | Low conversion efficiency | Rice straw/ultrasound-IL treatment, | Increased reducing sugar, delignification and cellulose conversion by 20.13–28.96%, 18.06–19.33% and 31.69–35.23%, respectively | [101] |
| Eucalyptus wood/ultrasound- distilled water (28 KHz, 300 W) | Effective disintegration of biomass with 35.5% increase in crystallinity | [64] | |||
| Microwave pretreatment | Continuous operation, less process time and low energy input | Requires high temperature for processing, no hot spots, low efficiency for apolar materials | Brachiara mutica (paragrass)/microwave-alkali (5% w/w), 120 °C, 30 min. | Increased total reducing sugars to 137.3% | [69] |
| Rice straw/microwave radiation for 4 min, at 190 °C) | High specific yield of methane (325.23 mL/g/VS) | [70] |
5. Enzymatic Hydrolysis as an Integral Step in Biorefineries
6. Bioconversion of Lignocellulosic Biomass into Biofuels and Value-Added Materials
6.1. Bioethanol
6.2. Biodiesel
6.3. Biomethane
6.4. 5-Hydroxymethylfurfural (HMF)
6.5. Biohydrogen
7. Environmental Sustainability of Biofuels and Value-Added Biochemicals from Lignocellulosics
8. Current Challenges and Future Prospects
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, V.; Tsai, M.-L.; Chen, C.-W.; Sun, P.-P.; Patel, A.K.; Singhania, R.R.; Nargotra, P.; Dong, C.-D. Deep eutectic solvents as promising pretreatment agents for sustainable lignocellulosic biorefineries: A review. Bioresour. Technol. 2022, 360, 127631. [Google Scholar] [CrossRef]
- Devi, A.; Bajar, S.; Kour, H.; Kothari, R.; Pant, D.; Singh, A. Lignocellulosic Biomass Valorization for Bioethanol Production: A Circular Bioeconomy Approach. BioEnergy Res. 2022, 15, 1820–1841. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.-L.; Nargotra, P.; Chen, C.-W.; Sun, P.-P.; Singhania, R.R.; Patel, A.K.; Dong, C.-D. Journey of lignin from a roadblock to bridge for lignocellulose biorefineries: A comprehensive review. Sci. Total. Environ. 2022, 863, 160560. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.-L.; Nargotra, P.; Chen, C.-W.; Kuo, C.-H.; Sun, P.-P.; Dong, C.-D. Agro-Industrial Food Waste as a Low-Cost Substrate for Sustainable Production of Industrial Enzymes: A Critical Review. Catalysts 2022, 12, 1373. [Google Scholar] [CrossRef]
- Weng, Z.-H.; Nargotra, P.; Kuo, C.-H.; Liu, Y.-C. Immobilization of Recombinant Endoglucanase (CelA) from Clostridium thermocellum on Modified Regenerated Cellulose Membrane. Catalysts 2022, 12, 1356. [Google Scholar] [CrossRef]
- Periyasamy, S.; Isabel, J.B.; Kavitha, S.; Karthik, V.; Mohamed, B.A.; Gizaw, D.G.; Sivashanmugam, P.; Aminabhavi, T.M. Recent advances in consolidated bioprocessing for conversion of lignocellulosic biomass into bioethanol—A review. Chem. Eng. J. 2022, 453, 139783. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef] [Green Version]
- Razzak, S.A.; Lucky, R.A.; Hossain, M.M.; Delasa, H. Valorization of Microalgae Biomass to Biofuel Production: A review. Energy Nexus 2022, 7, 100139. [Google Scholar] [CrossRef]
- Sharma, V.; Nargotra, P.; Sharma, S.; Bajaj, B.K. Efficacy and functional mechanisms of a novel combinatorial pretreatment approach based on deep eutectic solvent and ultrasonic waves for bioconversion of sugarcane bagasse. Renew. Energy 2020, 163, 1910–1922. [Google Scholar] [CrossRef]
- Sharma, V.; Nargotra, P.; Sharma, S.; Bajaj, B.K. Efficient bioconversion of sugarcane tops biomass into biofuel-ethanol using an optimized alkali-ionic liquid pretreatment approach. Biomass Convers. Biorefin. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Sharma, V.; Bhat, B.; Gupta, M.; Vaid, S.; Sharma, S.; Nargotra, P.; Singh, S.; Bajaj, B.K. Role of Systematic Biology in Biorefining of Lignocellulosic Residues for Biofuels and Chemicals Production. In Sustainable Biotechnology—Enzymatic Resources of Renewable Energy; Singh, O.V., Chandel, A.K., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 5–55. ISBN 978-3-319-95480-6. [Google Scholar]
- Banu, J.R.; Preethi; Kavitha, S.; Tyagi, V.K.; Gunasekaran, M.; Karthikeyan, O.P.; Kumar, G. Lignocellulosic biomass based biorefinery: A successful platform towards circular bioeconomy. Fuel 2021, 302, 121086. [Google Scholar] [CrossRef]
- Vu, H.P.; Nguyen, L.N.; Vu, M.T.; Johir, M.A.H.; McLaughlan, R.; Nghiem, L.D. A comprehensive review on the framework to valorise lignocellulosic biomass as biorefinery feedstocks. Sci. Total Environ. 2020, 743, 140630. [Google Scholar] [CrossRef]
- Sharma, S.; Nargotra, P.; Sharma, V.; Bangotra, R.; Kaur, M.; Kapoor, N.; Paul, S.; Bajaj, B.K. Nanobiocatalysts for efficacious bioconversion of ionic liquid pretreated sugarcane tops biomass to biofuel. Bioresour. Technol. 2021, 333, 125191. [Google Scholar] [CrossRef]
- Nargotra, P.; Sharma, V.; Bajaj, B.K. Consolidated bioprocessing of surfactant-assisted ionic liquid-pretreated Parthenium hysterophorus L. biomass for bioethanol production. Bioresour. Technol. 2019, 289, 121611. [Google Scholar] [CrossRef]
- Nargotra, P.; Sharma, V.; Sharma, S.; Kapoor, N.; Bajaj, B.K. Development of consolidated bioprocess for biofuel-ethanol production from ultrasound-assisted deep eutectic solvent pretreated Parthenium hysterophorus biomass. Biomass Convers. Biorefin. 2020, 12, 5767–5782. [Google Scholar] [CrossRef]
- Vaid, S.; Nargotra, P.; Bajaj, B.K. Consolidated bioprocessing for biofuel-ethanol production from pine needle biomass. Environ. Prog. Sustain. Energy 2018, 37, 546–552. [Google Scholar] [CrossRef]
- Sharma, V.; Nargotra, P.; Bajaj, B.K. Ultrasound and surfactant assisted ionic liquid pretreatment of sugarcane bagasse for enhancing saccharification using enzymes from an ionic liquid tolerant Aspergillus assiutensis VS34. Bioresour. Technol. 2019, 285, 121319. [Google Scholar] [CrossRef]
- Vaid, S.; Bhat, N.; Nargotra, P.; Bajaj, B.K. Combinatorial application of ammonium carbonate and sulphuric acid pretreatment to achieve enhanced sugar yield from pine needle biomass for potential biofuel–ethanol production. Energy Ecol. Environ. 2018, 3, 126–135. [Google Scholar] [CrossRef]
- Hazeena, S.H.; Sindhu, R.; Pandey, A.; Binod, P. Lignocellulosic bio-refinery approach for microbial 2,3-Butanediol production. Bioresour. Technol. 2020, 302, 122873. [Google Scholar] [CrossRef]
- Sharma, H.K.; Xu, C.; Qin, W. Biological Pretreatment of Lignocellulosic Biomass for Biofuels and Bioproducts: An Overview. Waste Biomass Valoriz. 2019, 10, 235–251. [Google Scholar] [CrossRef]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Haq, I.U.; Qaisar, K.; Nawaz, A.; Akram, F.; Mukhtar, H.; Zohu, X.; Xu, Y.; Mumtaz, M.W.; Rashid, U.; Ghani, W.A.W.A.K.; et al. Advances in Valorization of Lignocellulosic Biomass towards Energy Generation. Catalysts 2021, 11, 309. [Google Scholar] [CrossRef]
- Kumar, V.; Patel, S.K.S.; Gupta, R.K.; Otari, S.V.; Gao, H.; Lee, J.; Zhang, L. Enhanced Saccharification and Fermentation of Rice Straw by Reducing the Concentration of Phenolic Compounds Using an Immobilized Enzyme Cocktail. Biotechnol. J. 2019, 14, e1800468. [Google Scholar] [CrossRef] [PubMed]
- Manisha; Yadav, S.K. Technological advances and applications of hydrolytic enzymes for valorization of lignocellulosic biomass. Bioresour. Technol. 2017, 245, 1727–1739. [Google Scholar] [CrossRef] [PubMed]
- Wicker, R.J.; Kumar, G.; Khan, E.; Bhatnagar, A. Emergent green technologies for cost-effective valorization of microalgal biomass to renewable fuel products under a biorefinery scheme. Chem. Eng. J. 2021, 415, 128932. [Google Scholar] [CrossRef]
- Toor, M.; Kumar, S.S.; Malyan, S.K.; Bishnoi, N.R.; Mathimani, T.; Rajendran, K.; Pugazhendhi, A. An overview on bioethanol production from lignocellulosic feedstocks. Chemosphere 2020, 242, 125080. [Google Scholar] [CrossRef]
- Elegbede, J.; Ajayi, V.; Lateef, A. Microbial valorization of corncob: Novel route for biotechnological products for sustainable bioeconomy. Environ. Technol. Innov. 2021, 24, 102073. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Sarsaiya, S.; Patel, A.; Juneja, A.; Singh, R.P.; Yan, B.; Awasthi, S.K.; Jain, A.; Liu, T.; Duan, Y.; et al. Refining biomass residues for sustainable energy and bio-products: An assessment of technology, its importance, and strategic applications in circular bio-economy. Renew. Sustain. Energy Rev. 2020, 127, 109876. [Google Scholar] [CrossRef]
- Velvizhi, G.; Balakumar, K.; Shetti, N.P.; Ahmad, E.; Pant, K.K.; Aminabhavi, T.M. Integrated biorefinery processes for conversion of lignocellulosic biomass to value added materials: Paving a path towards circular economy. Bioresour. Technol. 2022, 343, 126151. [Google Scholar] [CrossRef]
- A Linares-Pasten, J.; Andersson, M.; NKarlsson, E. Thermostable Glycoside Hydrolases in Biorefinery Technologies. Cur. Biotechnol. 2014, 3, 26–44. [Google Scholar] [CrossRef]
- Jin, Q.; Yang, L.; Poe, N.; Huang, H. Integrated processing of plant-derived waste to produce value-added products based on the biorefinery concept. Trends Food Sci. Technol. 2018, 74, 119–131. [Google Scholar] [CrossRef]
- Vaid, S.; Sharma, S.; Bajaj, B.K. Chemo-enzymatic approaches for consolidated bioconversion of Saccharum spontaneum biomass to ethanol-biofuel. Bioresour. Technol. 2021, 329, 124898. [Google Scholar] [CrossRef]
- Wagle, A.; Angove, M.J.; Mahara, A.; Wagle, A.; Mainali, B.; Martins, M.; Goldbeck, R.; Paudel, S.R. Multi-stage pre-treatment of lignocellulosic biomass for multi-product biorefinery: A review. Sustain. Energy Technol. Assess. 2022, 49, 101702. [Google Scholar] [CrossRef]
- Nargotra, P.; Sharma, V.; Gupta, M.; Kour, S.; Bajaj, B.K. Application of ionic liquid and alkali pretreatment for enhancing saccharification of sunflower stalk biomass for potential biofuel-ethanol production. Bioresour. Technol. 2018, 267, 560–568. [Google Scholar] [CrossRef]
- Houfani, A.A.; Anders, N.; Spiess, A.C.; Baldrian, P.; Benallaoua, S. Insights from enzymatic degradation of cellulose and hemicellulose to fermentable sugars—A review. Biomass Bioenergy 2020, 134, 105481. [Google Scholar] [CrossRef]
- Alayoubi, R.; Mehmood, N.; Husson, E.; Kouzayha, A.; Tabcheh, M.; Chaveriat, L.; Sarazin, C.; Gosselin, I. Low temperature ionic liquid pretreatment of lignocellulosic biomass to enhance bioethanol yield. Renew. Energy 2020, 145, 1808–1816. [Google Scholar] [CrossRef]
- Li, H.-Y.; Chen, X.; Wang, C.-Z.; Sun, S.-N.; Sun, R.-C. Evaluation of the two-step treatment with ionic liquids and alkali for enhancing enzymatic hydrolysis of Eucalyptus: Chemical and anatomical changes. Biotechnol. Biofuels 2016, 9, 166. [Google Scholar] [CrossRef] [Green Version]
- Kohli, K.; Katuwal, S.; Biswas, A.; Sharma, B.K. Effective delignification of lignocellulosic biomass by microwave assisted deep eutectic solvents. Bioresour. Technol. 2020, 303, 122897. [Google Scholar] [CrossRef]
- Zulkefli, S.; Abdulmalek, E.; Rahman, M.B.A. Pretreatment of oil palm trunk in deep eutectic solvent and optimization of enzymatic hydrolysis of pretreated oil palm trunk. Renew. Energy 2017, 107, 36–41. [Google Scholar] [CrossRef]
- Haykir, N.I.; Soysal, K.; Yaglikci, S.; Gokce, Y. Assessing the effect of protic ionic liquid pretreatment of Pinus radiata from different perspectives including solvent-water ratio. J. Wood Chem. Technol. 2021, 41, 236–248. [Google Scholar] [CrossRef]
- Klímek, P.; Meinlschmidt, P.; Wimmer, R.; Plinke, B.; Schirp, A. Using sunflower (Helianthus annuus L.), topinambour (Helianthus tuberosus L.) and cup-plant (Silphium perfoliatum L.) stalks as alternative raw materials for particleboards. Ind. Crop. Prod. 2016, 92, 157–164. [Google Scholar] [CrossRef]
- Paul, S.K.; Chakraborty, S. Microwave-assisted ionic liquid-mediated rapid catalytic conversion of non-edible lignocellulosic Sunn hemp fibres to biofuels. Bioresour. Technol. 2018, 253, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Fakayode, O.A.; Akpabli-Tsigbe, N.D.K.; Wahia, H.; Tu, S.; Ren, M.; Zhou, C.; Ma, H. Integrated bioprocess for bio-ethanol production from watermelon rind biomass: Ultrasound-assisted deep eutectic solvent pretreatment, enzymatic hydrolysis and fermentation. Renew. Energy 2021, 180, 258–270. [Google Scholar] [CrossRef]
- Ge, S.; Wu, Y.; Peng, W.; Xia, C.; Mei, C.; Cai, L.; Shi, S.Q.; Sonne, C.; Lam, S.S.; Tsang, Y.F. High-pressure CO2 hydrothermal pretreatment of peanut shells for enzymatic hydrolysis conversion into glucose. Chem. Eng. J. 2020, 385, 123949. [Google Scholar] [CrossRef]
- Łukajtis, R.; Rybarczyk, P.; Kucharska, K.; Konopacka-Łyskawa, D.; Słupek, E.; Wychodnik, K.; Kamiński, M. Optimization of Saccharification Conditions of Lignocellulosic Biomass under Alkaline Pre-Treatment and Enzymatic Hydrolysis. Energies 2018, 11, 886. [Google Scholar] [CrossRef] [Green Version]
- Oliva, J.M.; Negro, M.J.; Manzanares, P.; Ballesteros, I.; Chamorro, M.Á.; Sáez, F.; Ballesteros, M.; Moreno, A.D. A Sequential Steam Explosion and Reactive Extrusion Pretreatment for Lignocellulosic Biomass Conversion within a Fermentation-Based Biorefinery Perspective. Fermentation 2017, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Pham, T.T.H.; Kim, T.H. Effects of Additional Xylanase on Saccharification and Ethanol Fermentation of Ammonia-Pretreated Corn Stover and Rice Straw. Energies 2020, 13, 4574. [Google Scholar] [CrossRef]
- Hoang, A.T.; Ong, H.C.; Fattah, I.M.R.; Chong, C.T.; Cheng, C.K.; Sakthivel, R.; Ok, Y.S. Progress on the lignocellulosic biomass pyrolysis for biofuel production toward environmental sustainability. Fuel Process. Technol. 2021, 223, 106997. [Google Scholar] [CrossRef]
- Siqueira, J.G.W.; Rodrigues, C.; Vandenberghe, L.P.D.S.; Woiciechowski, A.L.; Soccol, C.R. Current advances in on-site cellulase production and application on lignocellulosic biomass conversion to biofuels: A review. Biomass Bioenergy 2020, 132, 105419. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Chemistry and Specialty Industrial Applications of Lignocellulosic Biomass. Waste Biomass Valoriz. 2021, 12, 2145–2169. [Google Scholar] [CrossRef]
- Haldar, D.; Dey, P.; Patel, A.K.; Dong, C.-D.; Singhania, R.R. A Critical Review on the Effect of Lignin Redeposition on Biomass in Controlling the Process of Enzymatic Hydrolysis. BioEnergy Res. 2022, 15, 863–874. [Google Scholar] [CrossRef]
- Hasanov, I.; Raud, M.; Kikas, T. The Role of Ionic Liquids in the Lignin Separation from Lignocellulosic Biomass. Energies 2020, 13, 4864. [Google Scholar] [CrossRef]
- Lobato-Peralta, D.R.; Duque-Brito, E.; Villafán-Vidales, H.I.; Longoria, A.; Sebastian, P.; Cuentas-Gallegos, A.K.; Arancibia-Bulnes, C.A.; Okoye, P.U. A review on trends in lignin extraction and valorization of lignocellulosic biomass for energy applications. J. Clean. Prod. 2021, 293, 126123. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Raj, T.; Chen, C.-W.; Ponnusamy, V.K.; Tahir, N.; Kim, S.-H.; Dong, C.-D. Lignin valorisation via enzymes: A sustainable approach. Fuel 2022, 311, 122608. [Google Scholar] [CrossRef]
- Nanda, S.; Mohanty, P.; Pant, K.K.; Naik, S.; Kozinski, J.A.; Dalai, A.K. Characterization of North American Lignocellulosic Biomass and Biochars in Terms of their Candidacy for Alternate Renewable Fuels. BioEnergy Res. 2013, 6, 663–677. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G.; Morgan, T.J. An overview of the organic and inorganic phase composition of biomass. Fuel 2012, 94, 1–33. [Google Scholar] [CrossRef]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Patel, A.K.; Pant, D.; Banu, J.R.; Rao, C.V.; Kim, Y.-G.; Yang, Y.-H. Recent developments in pretreatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresour. Technol. 2020, 300, 122724. [Google Scholar] [CrossRef]
- Jatoi, A.S.; Abbasi, S.A.; Hashmi, Z.; Shah, A.K.; Alam, M.S.; Bhatti, Z.A.; Maitlo, G.; Hussain, S.; Khandro, G.A.; Usto, M.A.; et al. Recent trends and future perspectives of lignocellulose biomass for biofuel production: A comprehensive review. Biomass Convers. Biorefin. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Nargotra, P.; Vaid, S.; Bajaj, B.K. Cellulase Production from Bacillus subtilis SV1 and Its Application Potential for Saccharification of Ionic Liquid Pretreated Pine Needle Biomass under One Pot Consolidated Bioprocess. Fermentation 2016, 2, 19. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Rawat, R.; Oberoi, H.S.; Ramteke, P.W. A Review on Fuel Ethanol Production From Lignocellulosic Biomass. Int. J. Green Energy 2015, 12, 949–960. [Google Scholar] [CrossRef]
- He, Z.; Wang, Z.; Zhao, Z.; Yi, S.; Mu, J.; Wang, X. Influence of ultrasound pretreatment on wood physiochemical structure. Ultrason. Sonochem. 2017, 34, 136–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subhedar, P.B.; Ray, P.; Gogate, P.R. Intensification of delignification and subsequent hydrolysis for the fermentable sugar production from lignocellulosic biomass using ultrasonic irradiation. Ultrason. Sonochem. 2018, 40, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Kunaver, M.; Jasiukaitytė, E.; Čuk, N. Ultrasonically assisted liquefaction of lignocellulosic materials. Bioresour. Technol. 2012, 103, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Haldar, D.; Purkait, M.K. A review on the environment-friendly emerging techniques for pretreatment of lignocellulosic biomass: Mechanistic insight and advancements. Chemosphere 2021, 264, 128523. [Google Scholar] [CrossRef] [PubMed]
- Hoang, A.T.; Nižetić, S.; Ong, H.C.; Mofijur, M.; Ahmed, S.; Ashok, B.; Bui, V.T.V.; Chau, M.Q. Insight into the recent advances of microwave pretreatment technologies for the conversion of lignocellulosic biomass into sustainable biofuel. Chemosphere 2021, 281, 130878. [Google Scholar] [CrossRef]
- Nuchdang, S.; Thongtus, V.; Khemkhao, M.; Kirdponpattara, S.; Moore, E.J.; Setiabudi, H.D.B.; Phalakornkule, C. Enhanced production of reducing sugars from paragrass using microwave-assisted alkaline pretreatment. Biomass Convers. Biorefin. 2021, 11, 2471–2483. [Google Scholar] [CrossRef]
- Kainthola, J.; Shariq, M.; Kalamdhad, A.; Goud, V.V. Enhanced methane potential of rice straw with microwave assisted pretreatment and its kinetic analysis. J. Environ. Manag. 2019, 232, 188–196. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Y.; Yang, X.; McQueen-Mason, S.J.; Gomez, L.D.; Macquarrie, D.J. Comparative evaluation of microwave-assisted acid, alkaline, and inorganic salt pretreatments of sugarcane bagasse for sugar recovery. Biomass Convers. Biorefin. 2021, 11, 2681–2693. [Google Scholar] [CrossRef]
- Kundu, C.; Samudrala, S.P.; Kibria, M.A.; Bhattacharya, S. One-step peracetic acid pretreatment of hardwood and softwood biomass for platform chemicals production. Sci. Rep. 2021, 11, 11183. [Google Scholar] [CrossRef]
- Morais, W.G.; Pacheco, T.F.; Corrêa, P.S.; Martins, A.A.; Mata, T.M.; Caetano, N.S. Acid pretreatment of sugarcane biomass to obtain hemicellulosic hydrolisate rich in fermentable sugar. Energy Rep. 2020, 6, 18–23. [Google Scholar] [CrossRef]
- Mehrez, I.; Chandrasekhar, K.; Kim, W.; Kim, S.-H.; Kumar, G. Comparison of alkali and ionic liquid pretreatment methods on the biochemical methane potential of date palm waste biomass. Bioresour. Technol. 2022, 360, 127505. [Google Scholar] [CrossRef]
- Goshadrou, A. Bioethanol production from Cogongrass by sequential recycling of black liquor and wastewater in a mild-alkali pretreatment. Fuel 2019, 258, 116141. [Google Scholar] [CrossRef]
- Woiciechowski, A.L.; Neto, C.J.D.; de Souza Vandenberghe, L.P.; de Carvalho Neto, D.P.; Sydney, A.C.N.; Letti, L.A.J.; Karp, S.G.; Torres, L.A.Z.; Soccol, C.R. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance—Conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef]
- Dotsenko, A.S.; Dotsenko, G.S.; Senko, O.V.; Stepanov, N.A.; Lyagin, I.V.; Efremenko, E.N.; Gusakov, A.V.; Zorov, I.N.; Rubtsova, E.A. Complex effect of lignocellulosic biomass pretreatment with 1-butyl-3-methylimidazolium chloride ionic liquid on various aspects of ethanol and fumaric acid production by immobilized cells within SSF. Bioresour. Technol. 2018, 250, 429–438. [Google Scholar] [CrossRef]
- Lim, W.-L.; Gunny, A.A.N.; Kasim, F.H.; AlNashef, I.M.; Arbain, D. Alkaline deep eutectic solvent: A novel green solvent for lignocellulose pulping. Cellulose 2019, 26, 4085–4098. [Google Scholar] [CrossRef]
- Amesho, K.T.T.; Lin, Y.-C.; Mohan, S.V.; Halder, S.; Ponnusamy, V.K.; Jhang, S.-R. Deep eutectic solvents in the transformation of biomass into biofuels and fine chemicals: A review. Environ. Chem. Lett. 2022, 20, 1–48. [Google Scholar] [CrossRef]
- Jing, Y.; Li, F.; Li, Y.; Jiang, D.; Lu, C.; Zhang, Z.; Zhang, Q. Biohydrogen production by deep eutectic solvent delignification-driven enzymatic hydrolysis and photo-fermentation: Effect of liquid–solid ratio. Bioresour. Technol. 2022, 349, 126867. [Google Scholar] [CrossRef]
- Tan, Y.T.; Ngoh, G.C.; Chua, A.S.M. Effect of functional groups in acid constituent of deep eutectic solvent for extraction of reactive lignin. Bioresour. Technol. 2019, 281, 359–366. [Google Scholar] [CrossRef]
- Thi, S.; Lee, K.M. Comparison of deep eutectic solvents (DES) on pretreatment of oil palm empty fruit bunch (OPEFB): Cellulose digestibility, structural and morphology changes. Bioresour. Technol. 2019, 282, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.A.; Murton, K.D.; Smith, D.A.; Dedual, G. A review on organosolv pretreatment of softwood with a focus on enzymatic hydrolysis of cellulose. Biomass Convers. Biorefin. 2022, 12, 5427–5442. [Google Scholar] [CrossRef]
- Borand, M.N.; Karaosmanoğlu, F. Effects of organosolv pretreatment conditions for lignocellulosic biomass in biorefinery applications: A review. J. Renew. Sustain. Energy 2018, 10, 033104. [Google Scholar] [CrossRef]
- Karnaouri, A.; Asimakopoulou, G.; Kalogiannis, K.G.; Lappas, A.; Topakas, E. Efficient d-lactic acid production by Lactobacillus delbrueckii subsp. bulgaricus through conversion of organosolv pretreated lignocellulosic biomass. Biomass Bioenergy 2020, 140, 105672. [Google Scholar] [CrossRef]
- Joy, S.P.; Krishnan, C. Modified organosolv pretreatment for improved cellulosic ethanol production from sorghum biomass. Ind. Crop. Prod. 2022, 177, 114409. [Google Scholar] [CrossRef]
- Tang, C.; Shan, J.; Chen, Y.; Zhong, L.; Shen, T.; Zhu, C.; Ying, H. Organic amine catalytic organosolv pretreatment of corn stover for enzymatic saccharification and high-quality lignin. Bioresour. Technol. 2017, 232, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, Y.; Kong, W.; Wei, J.; Song, W.; Wang, S. Improving enzymatic hydrolysis of lignocellulosic biomass by bio-coordinated physicochemical pretreatment—A review. Energy Rep. 2022, 8, 696–709. [Google Scholar] [CrossRef]
- Mockaitis, G.; Bruant, G.; Foresti, E.; Zaiat, M.; Guiot, S.R. Physicochemical pretreatment selects microbial communities to produce alcohols through metabolism of volatile fatty acids. Biomass Convers. Biorefin. 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Raud, M.; Kikas, T.; Sippula, O.; Shurpali, N. Potentials and challenges in lignocellulosic biofuel production technology. Renew. Sustain. Energy Rev. 2019, 111, 44–56. [Google Scholar] [CrossRef]
- Kuglarz, M.; Gunnarsson, I.B.; Svensson, S.-E.; Prade, T.; Johansson, E.; Angelidaki, I. Ethanol production from industrial hemp: Effect of combined dilute acid/steam pretreatment and economic aspects. Bioresour. Technol. 2014, 163, 236–243. [Google Scholar] [CrossRef]
- Vaid, S.; Sharma, S.; Dutt, H.C.; Mahajan, R.; Bajaj, B.K. One pot consolidated bioprocess for conversion of Saccharum spontaneum biomass to ethanol-biofuel. Energy Convers. Manag. 2021, 250, 114880. [Google Scholar] [CrossRef]
- Hou, X.; Wang, Z.; Sun, J.; Li, M.; Wang, S.; Chen, K.; Gao, Z. A microwave-assisted aqueous ionic liquid pretreatment to enhance enzymatic hydrolysis of Eucalyptus and its mechanism. Bioresour. Technol. 2019, 272, 99–104. [Google Scholar] [CrossRef]
- Hosseini Koupaie, E.; Dahadha, S.; Bazyar Lakeh, A.A.; Azizi, A.; Elbeshbishy, E. Enzymatic pretreatment of lignocellulosic biomass for enhanced biomethane production—A review. J. Environ. Manag. 2019, 233, 774–784. [Google Scholar] [CrossRef]
- Prasad, R.K.; Chatterjee, S.; Mazumder, P.B.; Gupta, S.K.; Sharma, S.; Vairale, M.G.; Datta, S.; Dwivedi, S.K.; Gupta, D.K. Bioethanol production from waste lignocelluloses: A review on microbial degradation potential. Chemosphere 2019, 231, 588–606. [Google Scholar] [CrossRef]
- Ni Sun, S.; Chen, X.; Tao, Y.H.; Cao, X.F.; Li, M.F.; Wen, J.L.; Nie, S.X.; Sun, R.C. Pretreatment of Eucalyptus urophylla in γ-valerolactone/dilute acid system for removal of non-cellulosic components and acceleration of enzymatic hydrolysis. Ind. Crop. Prod. 2019, 132, 21–28. [Google Scholar] [CrossRef]
- Jiang, D.; Ge, X.; Zhang, T.; Chen, Z.; Zhang, Z.; He, C.; Zhang, Q.; Li, Y. Effect of alkaline pretreatment on photo-fermentative hydrogen production from giant reed: Comparison of NaOH and Ca(OH)2. Bioresour. Technol. 2020, 304, 123001. [Google Scholar] [CrossRef]
- Das, L.; Achinivu, E.C.; Barcelos, C.A.; Sundstrom, E.; Amer, B.; Baidoo, E.E.K.; Simmons, B.A.; Sun, N.; Gladden, J.M. Deconstruction of Woody Biomass via Protic and Aprotic Ionic Liquid Pretreatment for Ethanol Production. ACS Sustain. Chem. Eng. 2021, 9, 4422–4432. [Google Scholar] [CrossRef]
- Smuga-Kogut, M.; Szymanowska-Powałowska, D.; Markiewicz, R.; Piskier, T.; Kogut, T. Ionic liquid pretreatment of stinging nettle stems and giant miscanthus for bioethanol production. Sci. Rep. 2021, 11, 18465. [Google Scholar] [CrossRef]
- Manivannan, H.; Anguraj, B.L. Valorization of fruit waste using DES pretreatment and hydrolysis over a heterogeneous catalyst for bioethanol production. Biomass Convers. Biorefin. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, J.; Wang, Y.; Sun, J.; Huang, P.; Chang, K. Effect of ultrasound on ionic liquid-hydrochloric acid pretreatment with rice straw. Biomass Convers. Biorefin. 2021, 11, 1749–1757. [Google Scholar] [CrossRef]
- Roth, J.C.G.; Hoeltz, M.; Benitez, L.B. Current approaches and trends in the production of microbial cellulases using residual lignocellulosic biomass: A bibliometric analysis of the last 10 years. Arch. Microbiol. 2020, 202, 935–951. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, V.; Nargotra, P.; Bajaj, B.K. Process desired functional attributes of an endoxylanase of GH10 family from a new strain of Aspergillus terreus S9. Int. J. Biol. Macromol. 2018, 115, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, V.; Nargotra, P.; Bajaj, B.K. Bioprocess development for production of a process-apt xylanase with multifaceted application potential for a range of industrial processes. SN Appl. Sci. 2020, 2, 739. [Google Scholar] [CrossRef] [Green Version]
- Madadi, M.; Tu, Y.; Abbas, A. Recent Status on Enzymatic Saccharification of Lignocellulosic Biomass for Bioethanol Production. Electron J. Biotechnol. 2017, 13, 135–143. [Google Scholar]
- Alokika; Singh, B. Enhanced production of bacterial xylanase and its utility in saccharification of sugarcane bagasse. Bioprocess Biosyst. Eng. 2020, 43, 1081–1091. [Google Scholar] [CrossRef]
- Cui, L.; Wang, Z.; Zeng, Y.; Yang, N.; Liu, M.; Zhao, Y.; Zheng, Y. Lignin Biodegradation and Its Valorization. Fermentation 2022, 8, 366. [Google Scholar] [CrossRef]
- Obeng, E.M.; Budiman, C.; Ongkudon, C.M. Identifying additives for cellulase enhancement—A systematic approach. Biocatal. Agric. Biotechnol. 2017, 11, 67–74. [Google Scholar] [CrossRef]
- Cai, C.; Qiu, X.; Zeng, M.; Lin, M.; Lin, X.; Lou, H.; Zhan, X.; Pang, Y.; Huang, J.; Xie, L. Using polyvinylpyrrolidone to enhance the enzymatic hydrolysis of lignocelluloses by reducing the cellulase non-productive adsorption on lignin. Bioresour. Technol. 2017, 227, 74–81. [Google Scholar] [CrossRef]
- Rocha-Martín, J.; Martinez-Bernal, C.; Pérez-Cobas, Y.; Reyes-Sosa, F.M.; García, B.D. Additives enhancing enzymatic hydrolysis of lignocellulosic biomass. Bioresour. Technol. 2017, 244, 48–56. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Biswas, J.K.; Da Silva, S.S. Emerging role of nanobiocatalysts in hydrolysis of lignocellulosic biomass leading to sustainable bioethanol production. Catal. Rev. 2019, 61, 1–26. [Google Scholar] [CrossRef]
- Grewal, J.; Ahmad, R.; Khare, S. Development of cellulase-nanoconjugates with enhanced ionic liquid and thermal stability for in situ lignocellulose saccharification. Bioresour. Technol. 2017, 242, 236–243. [Google Scholar] [CrossRef]
- Cheng, G.; Xing, J.; Pi, Z.; Liu, S.; Liu, Z.; Song, F. α-Glucosidase immobilization on functionalized Fe3O4 magnetic nanoparticles for screening of enzyme inhibitors. Chin. Chem. Lett. 2019, 30, 656–659. [Google Scholar] [CrossRef]
- Hossain, N.; Mahlia, T.M.I.; Saidur, R. Latest development in microalgae-biofuel production with nano-additives. Biotechnol. Biofuels 2019, 12, 125. [Google Scholar] [CrossRef] [Green Version]
- Karnaouri, A.; Muraleedharan, M.N.; Dimarogona, M.; Topakas, E.; Rova, U.; Sandgren, M.; Christakopoulos, P. Recombinant expression of thermostable processive MtEG5 endoglucanase and its synergism with MtLPMO from Myceliophthora thermophila during the hydrolysis of lignocellulosic substrates. Biotechnol. Biofuels 2017, 10, 126. [Google Scholar] [CrossRef]
- Vijayalakshmi, S.; Govindarajan, M.; Al-Mulahim, N.; Ahmed, Z.; Mahboob, S. Cellulase immobilized magnetic nanoparticles for green energy production from Allamanda schottii L.: Sustainability research in waste recycling. Saudi J. Biol. Sci. 2021, 28, 901–910. [Google Scholar] [CrossRef]
- Hwangbo, M.; Tran, J.L.; Chu, K.-H. Effective one-step saccharification of lignocellulosic biomass using magnetite-biocatalysts containing saccharifying enzymes. Sci. Total Environ. 2019, 647, 806–813. [Google Scholar] [CrossRef]
- Yan, J. Handbook of Clean Energy Systems; 6 Volume Set; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 978-1-118-38858-7. [Google Scholar]
- Sharma, S.; Sharma, P.; Sharma, V.; Bajaj, B.K. Polyhydroxybutyrate as an Eco-Friendly Alternative of Synthetic Plastics. In Environmental and Agricultural Microbiology; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 101–149. ISBN 978-1-119-52589-9. [Google Scholar]
- Azhar, S.H.M.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Faik, A.A.M.; Rodrigues, K.F. Yeasts in sustainable bioethanol production: A review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar] [CrossRef]
- Ramachandra, T.; Hebbale, D. Bioethanol from macroalgae: Prospects and challenges. Renew. Sustain. Energy Rev. 2020, 117, 109479. [Google Scholar] [CrossRef]
- Hossain, S.; Theodoropoulos, C.; Yousuf, A. Techno-economic evaluation of heat integrated second generation bioethanol and furfural coproduction. Biochem. Eng. J. 2019, 144, 89–103. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Alsaffar, M.A.; Mustapa, S.I. An overview of integration opportunities for sustainable bioethanol production from first- and second-generation sugar-based feedstocks. J. Clean. Prod. 2020, 245, 118857. [Google Scholar] [CrossRef]
- Jin, Y.; Shi, Z.; Xu, G.; Yang, H.; Yang, J. A stepwise pretreatment of sugarcane bagasse by alkaline and hydroxymethyl reagent for bioethanol production. Ind. Crop. Prod. 2020, 145, 112136. [Google Scholar] [CrossRef]
- Wang, H.; Peng, X.; Zhang, H.; Yang, S.; Li, H. Microorganisms-promoted biodiesel production from biomass: A review. Energy Convers. Manag. X 2021, 12, 100137. [Google Scholar] [CrossRef]
- Yusuf, N.; Kamarudin, S.; Yaakub, Z. Overview on the current trends in biodiesel production. Energy Convers. Manag. 2011, 52, 2741–2751. [Google Scholar] [CrossRef]
- Wang, H.; Rehman, K.U.; Liu, X.; Yang, Q.; Zheng, L.; Li, W.; Cai, M.; Li, Q.; Zhang, J.; Yu, Z. Insect biorefinery: A green approach for conversion of crop residues into biodiesel and protein. Biotechnol. Biofuels 2017, 10, 304. [Google Scholar] [CrossRef] [Green Version]
- Bateni, H.; Karimi, K. Biodiesel production from castor plant integrating ethanol production via a biorefinery approach. Chem. Eng. Res. Des. 2016, 107, 4–12. [Google Scholar] [CrossRef]
- Preethi; Banu, J.R.; Kavitha, S.; Kannah, R.Y.; Varjani, S.; Gunasekaran, M. Mild hydrogen peroxide interceded bacterial disintegration of waste activated sludge for efficient biomethane production. Sci. Total Environ. 2022, 817, 152873. [Google Scholar] [CrossRef]
- Okolie, J.A.; Mukherjee, A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Next-generation biofuels and platform biochemicals from lignocellulosic biomass. Int. J. Energy Res. 2021, 45, 14145–14169. [Google Scholar] [CrossRef]
- Kohli, K.; Prajapati, R.; Sharma, B.K. Bio-Based Chemicals from Renewable Biomass for Integrated Biorefineries. Energies 2019, 12, 233. [Google Scholar] [CrossRef] [Green Version]
- Gandini, A.; Belgacem, M.N. Recent Contributions to the Preparation of Polymers Derived from Renewable Resources. J. Polym. Environ. 2002, 10, 105–114. [Google Scholar] [CrossRef]
- Ruby, M.-P.; Schüth, F. Synthesis of N-alkyl-4-vinylpyridinium-based cross-linked polymers and their catalytic performance for the conversion of fructose into 5-hydroxymethylfurfural. Green Chem. 2016, 18, 3422–3429. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, C.; He, C.; Dai, Y.; Jia, X.; Yang, Y. Efficient dehydration of fructose to 5-hydroxymethylfurfural over sulfonated carbon sphere solid acid catalysts. Catal. Today 2016, 264, 123–130. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Rajendran, K.; Pugazhendhi, A.; Rao, C.V.; Atabani, A.; Kumar, G.; Yang, Y.-H. Renewable biohydrogen production from lignocellulosic biomass using fermentation and integration of systems with other energy generation technologies. Sci. Total. Environ. 2021, 765, 144429. [Google Scholar] [CrossRef]
- Mechery, J.; Thomas, D.M.; Kumar, C.S.P.; Joseph, L.; Sylas, V.P. Biohydrogen production from acidic and alkaline hydrolysates of paddy straw using locally isolated facultative bacteria through dark fermentation. Biomass-Convers. Biorefin. 2021, 11, 1263–1272. [Google Scholar] [CrossRef]
- Cieciura-Włoch, W.; Borowski, S.; Otlewska, A. Biohydrogen production from fruit and vegetable waste, sugar beet pulp and corn silage via dark fermentation. Renew. Energy 2020, 153, 1226–1237. [Google Scholar] [CrossRef]
- Irmak, S.; Meryemoglu, B.; Sandip, A.; Subbiah, J.; Mitchell, R.B.; Sarath, G. Microwave pretreatment effects on switchgrass and miscanthus solubilization in subcritical water and hydrolysate utilization for hydrogen production. Biomass Bioenergy 2018, 108, 48–54. [Google Scholar] [CrossRef]
- Prabha, S.P.; Nagappan, S.; Rathna, R.; Viveka, R.; Nakkeeran, E. 21—Blue Biotechnology: A Vision for Future Marine Biore-fineries. In Refining Biomass Residues for Sustainable Energy and Bioproducts; Kumar, R.P., Gnansounou, E., Raman, J.K., Baskar, G., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 463–480. ISBN 978-0-12-818996-2. [Google Scholar]
- Chang, W.-R.; Hwang, J.-J.; Wu, W. Environmental impact and sustainability study on biofuels for transportation applications. Renew. Sustain. Energy Rev. 2017, 67, 277–288. [Google Scholar] [CrossRef]
- Dehghanimadvar, M.; Aslani, A.; Ahmadi, M.H.; Ghomi, N.S.K. Current status and future forecasting of biofuels technology development. Int. J. Energy Res. 2019, 43, 1142–1160. [Google Scholar] [CrossRef]




| S. No. | Lignocellulose Feedstocks | Cellulose (%) | Hemicelluloses (%) | Lignin (%) | Reference |
|---|---|---|---|---|---|
| 1. | Sugarcane bagasse | 38.6 | 20 | 24.68 | [9] |
| 2. | Sunflower stalks | 27.22 | 11.94 | - | [35] |
| 3. | Oat flakes | 21 | 38% | 27 | [36] |
| 4. | Spruce sawdust | 55.4 | 1.4% arabinose, 4.2% xylose | 28.7 | [37] |
| 5. | Eucalyptus | 41.58 | 15.85 | 29.40 | [38] |
| 6. | Parthenium hysterophorus | 49.98 | 7.61%arabinose, 14.18% xylose | 17.6 | [15] |
| 7. | Saccharum spontaneum | 32.16 | 19.36 | 16.86 | [33] |
| 8. | Birchwood planks | 54.22 | 28.14 | 11.13 | [39] |
| 9. | Oak sawdust | 44.7 | 1.2% arabinose, 14.8% xylose | 26.7 | [37] |
| 10. | Oil palm trunk | 56.1 | 16.15 | 19.11 | [40] |
| 11. | Pine | 36.2 | 23.0 | 32.8 | [41] |
| 12. | Cup plant | 39 | 21 | 21 | [42] |
| 13. | Sun hemp fiber | 75.6 | 10.05 | 10.32 | [43] |
| 14. | Watermelon rind | 39.67 | 23.21 | 10.6 | [44] |
| 15. | Peanut shell | 36.9% glucan | 13.2% xylan, 1.5% galactan, 5.2% arabinan, 1.0% mannan, | 30.2% Klason lignin, 3.9% acid-soluble lignin, | [45] |
| 16. | Corn cob | 41 | 22.6 | 14.1 | [46] |
| 17. | Barley straw | 31.1 ± 0.8 | 27.2 ± 0.4 | 18.8 ± 0.2 | [47] |
| 18. | Corn stover | 31.5% glucan | 22.5% xylan, 2.1% galactan, 1.7% arabinan | 18 | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Tsai, M.-L.; Sharma, V.; Sun, P.-P.; Nargotra, P.; Bajaj, B.K.; Chen, C.-W.; Dong, C.-D. Environment Friendly Pretreatment Approaches for the Bioconversion of Lignocellulosic Biomass into Biofuels and Value-Added Products. Environments 2023, 10, 6. https://doi.org/10.3390/environments10010006
Sharma S, Tsai M-L, Sharma V, Sun P-P, Nargotra P, Bajaj BK, Chen C-W, Dong C-D. Environment Friendly Pretreatment Approaches for the Bioconversion of Lignocellulosic Biomass into Biofuels and Value-Added Products. Environments. 2023; 10(1):6. https://doi.org/10.3390/environments10010006
Chicago/Turabian StyleSharma, Surbhi, Mei-Ling Tsai, Vishal Sharma, Pei-Pei Sun, Parushi Nargotra, Bijender Kumar Bajaj, Chiu-Wen Chen, and Cheng-Di Dong. 2023. "Environment Friendly Pretreatment Approaches for the Bioconversion of Lignocellulosic Biomass into Biofuels and Value-Added Products" Environments 10, no. 1: 6. https://doi.org/10.3390/environments10010006
APA StyleSharma, S., Tsai, M. -L., Sharma, V., Sun, P. -P., Nargotra, P., Bajaj, B. K., Chen, C. -W., & Dong, C. -D. (2023). Environment Friendly Pretreatment Approaches for the Bioconversion of Lignocellulosic Biomass into Biofuels and Value-Added Products. Environments, 10(1), 6. https://doi.org/10.3390/environments10010006